|
Tofersen
Tofersen, sold under the brand name Qalsody, is a medication used for the treatment of amyotrophic lateral sclerosis (ALS). Tofersen is an antisense oligonucleotide that targets the production of superoxide dismutase 1, an enzyme whose mutant form is commonly associated with amyotrophic lateral sclerosis. It is administered as an intrathecal injection. The most common side effects include fatigue, arthralgia (joint pain), increased cerebrospinal (brain and spinal cord) fluid white blood cells, and myalgia (muscle pain). Tofersen was approved for medical use in the United States in April 2023, and in the European Union in May 2024. The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. Medical uses Tofersen is indicated to treat people with amyotrophic lateral sclerosis (ALS) associated with a mutation in the superoxide dismutase 1 (SOD1) gene (SOD1-ALS). History Tofersen was developed by Ionis Pharmaceuticals and was licensed to, and co-d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionis Pharmaceuticals
Ionis Pharmaceuticals, Inc. is a biotechnology company based in Carlsbad, California, that specializes in discovering and developing RNA-targeted therapeutics. The company has three commercially approved medicines: Spinraza ( Nusinersen), Tegsedi ( Inotersen), and Waylivra ( Volanesorsen), and has four drugs in pivotal studies: tominersen for Huntington's disease (together with Roche), tofersen for SOD1- ALS, AKCEA-APO(a)-LRx for cardiovascular disease, and AKCEA-TTR-LRx for all forms of TTR amyloidosis. The company was named Isis Pharmaceuticals until December 2015. History The company was founded in 1989 as Isis Pharmaceuticals by Stanley T. Crooke, a former head of research of GlaxoSmithKline, with the goal of commercializing antisense therapy. In 1992, the company received its first approval by the Food and Drug Administration for an investigational new drug application in 1992 for a genital warts drug candidate. The FDA approval marked the first time for the company t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
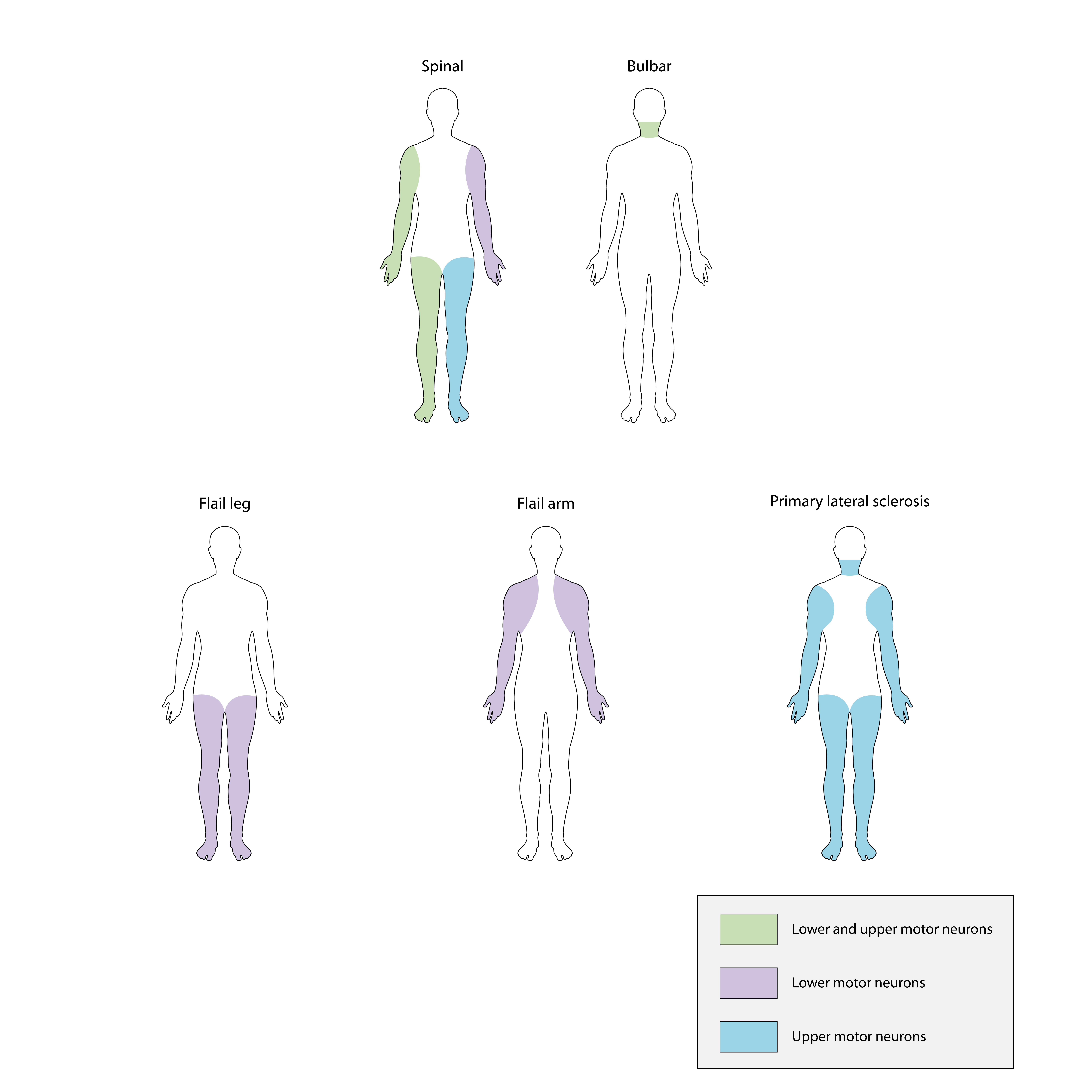
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS), also known as motor neuron disease (MND) or—in the United States—Lou Gehrig's disease (LGD), is a rare, Terminal illness, terminal neurodegenerative disease, neurodegenerative disorder that results in the progressive loss of both upper and lower motor neurons that normally control Skeletal muscle, voluntary muscle contraction. ALS is the most common form of the motor neuron diseases. ALS often presents in its early stages with gradual muscle Spasticity, stiffness, Fasciculation, twitches, Muscle weakness, weakness, and Muscle atrophy, wasting. Motor neuron loss typically continues until the abilities to eat, speak, move, and, lastly, breathe are all lost. While only 15% of people with ALS also fully develop frontotemporal dementia, an estimated 50% face at least some minor difficulties with cognitive disorder, thinking and behavior. Depending on which of the aforementioned symptoms develops first, ALS is classified as ''limb-onset'' (b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intrathecal
Intrathecal administration is a route of administration for drugs via an injection into the spinal canal, or into the subarachnoid space (sin. ''intrathecal space'') so that it reaches the cerebrospinal fluid (CSF). It is useful in several applications, such as for spinal anesthesia, chemotherapy, or pain management. This route is also used to introduce drugs that fight certain infections, particularly post-neurosurgical. Typically, the drug is given this way to avoid being stopped by the blood–brain barrier, as it may not be able to pass into the brain when given orally. Drugs given by the intrathecal route often have to be compounded specially by a pharmacist or technician because they cannot contain any preservative or other potentially harmful inactive ingredients that are sometimes found in standard injectable drug preparations. Intrathecal pseudodelivery is a technique where the drug is encapsulated in a porous capsule that is placed in communication with the CSF. In this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, pharmaceutical drug, drugs, medical nutrition therapy, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received institutional review board, health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small Pilot experiment, pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as the Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal product Medication (also called medicament, medicine, pharmaceutical drug, medicinal product, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the ...s for human use. See also * Committee for Medicinal Products for Veterinary Use References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The New York Times
''The New York Times'' (''NYT'') is an American daily newspaper based in New York City. ''The New York Times'' covers domestic, national, and international news, and publishes opinion pieces, investigative reports, and reviews. As one of the longest-running newspapers in the United States, the ''Times'' serves as one of the country's Newspaper of record, newspapers of record. , ''The New York Times'' had 9.13 million total and 8.83 million online subscribers, both by significant margins the List of newspapers in the United States, highest numbers for any newspaper in the United States; the total also included 296,330 print subscribers, making the ''Times'' the second-largest newspaper by print circulation in the United States, following ''The Wall Street Journal'', also based in New York City. ''The New York Times'' is published by the New York Times Company; since 1896, the company has been chaired by the Ochs-Sulzberger family, whose current chairman and the paper's publ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CNBC
CNBC is an American List of business news channels, business news channel owned by the NBCUniversal News Group, a unit of Comcast's NBCUniversal. The network broadcasts live business news and analysis programming during the morning, Daytime television in the United States, daytime trading day, and early-evening hours, with the remaining hours (such as weekday prime time and weekends) are filled by business-related Television documentary, documentaries and reality television programming, as well as occasional NBC Sports presentations. CNBC operates an accompanying financial news website, CNBC.com, which includes news articles, video and podcast content, as well as subscription-based services. CNBC's headquarters and main studios are located in Englewood Cliffs, New Jersey, while it also maintains a studio at the Nasdaq MarketSite in Times Square, New York City. CNBC was originally founded in April 1989 as the Consumer News and Business Channel, a joint venture between NBC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast track is one of five FDA approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and regenerative medicine advanced therapy. Fast track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical need. Serious condition: det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by the conditions. The conditions that orphan drugs are used to treat are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy that depends on the legislation (if there is any) of the country. Designation of a drug as an orphan drug has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug medical research, research and development. Examples of this can be that in the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |