|
Titanium Tetraazide
Titanium tetraazide is an inorganic chemical compound with the formula . It is a highly sensitive explosive, and has been prepared from titanium tetrafluoride and trimethylsilyl azide via the corresponding fluoride-azide exchange. Properties Titanium tetraazide has been characterized by vibrational spectroscopy and single-crystal X-ray diffraction. The compound was predicted in 2003 to be vibrationally stable, and was expected to have a tetrahedral structures containing linear bond angles, contrasting other metal azides which generally feature bent bond angles. After synthesis in 2004, the resulting titanium tetraazide did not exhibit linear bond angles, as the coordination numbers exceeded 4. References azide titanium Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Tetrafluoride
Titanium(IV) fluoride is the inorganic compound with the formula Ti F4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid. Preparation and structure The traditional method involves treatment of titanium tetrachloride with excess hydrogen fluoride: :TiCl4 + 4 HF → TiF4 + 4 HCl Purification is by sublimation, which involves reversible cracking of the polymeric structure. X-ray crystallography reveals that the Ti centres are octahedral, but conjoined in an unusual columnar structure. Reactions TiF4 forms adducts with many ligands. One example is the complex ''cis''-TiF4(CH3CN)2, which is formed by treatment with acetonitrile. It is also used as a reagent in the preparation of organofluorine compounds. With fluoride, the cluster i4F18sup>2- forms. It has an adamantane-like Ti4F6 core. Related to its Lewis acidity, TiF4 forms a variety of h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyl Azide
Trimethylsilyl azide is the organosilicon compound with the formula . A colorless liquid, it is a reagent in organic chemistry Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ..., serving as the equivalent of hydrazoic acid. Preparation Trimethylsilyl azide is commercially available. It may be prepared by the reaction of trimethylsilyl chloride and sodium azide: : Reactions The compound hydrolyzes to hydrazoic acid: : The compound adds to ketones and aldehydes to give the siloxy azides and subsequently tetrazoles: : It ring-opens epoxides to give azido alcohols. It has been used in the Oseltamivir total synthesis. Safety Trimethylsilyl azide is incompatible with moisture, strong oxidizing agents, and strong acids. Azides are often explosive, as illustrated by their u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
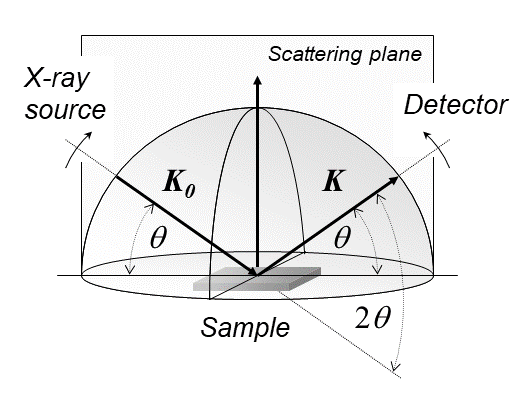
Vibrational Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. The resulting map of the directions of the X-rays far from the sample is called a diffraction pattern. It is different from X-ray crystallography which exploits X-ray diffraction to determine the arrangement of atoms in materials, and also has other components such as ways to map from experimental diffraction measurements to the positions of atoms. This article provides an overview of X-ray diffraction, starting with the early #History, history of x-rays and the discovery that they have the right spacings to be diffracted by crystals. In many cases these diffraction patterns can be #Introduction to x-ray diffraction theory, Interpreted using a single scattering or kinematical theory with conservation of energy (#Ewald's sphere, wave vecto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Compounds
The +4 oxidation state dominates titanium chemistry, but compounds in the +3 oxidation state are also numerous. Commonly, titanium adopts an octahedral coordination geometry in its complexes, but tetrahedral TiCl4 is a notable exception. Because of its high oxidation state, titanium(IV) compounds exhibit a high degree of covalent bonding. Oxides, sulfides, and alkoxides The most important oxide is TiO2, which exists in three important polymorphs; anatase, brookite, and rutile. All three are white diamagnetic solids, although mineral samples can appear dark (see rutile). They adopt polymeric structures in which Ti is surrounded by six oxide ligands that link to other Ti centers. The term '' titanates'' usually refers to titanium(IV) compounds, as represented by barium titanate (BaTiO3). With a perovskite structure, this material exhibits piezoelectric properties and is used as a transducer in the interconversion of sound and electricity. Many minerals are titanates, such as ilm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |