|
Thioredoxin Reductase
Thioredoxin reductases (TR, TrxR) () are enzymes that reduce thioredoxin (Trx). Two classes of thioredoxin reductase have been identified: one class in bacteria and some eukaryotes and one in animals. Bacterial TrxR also catalyzes the reduction of glutaredoxin like proteins known as NrdH. Both classes are flavoproteins which function as homodimers. Each monomer contains a flavin adenine dinucleotide, FAD prosthetic group, a NADPH binding domain, and an active site containing a redox-active disulfide bond. Cellular role Thioredoxin reductases are enzymes that catalyze the reduction of thioredoxin and hence they are a central component in the thioredoxin system. Together with thioredoxin (Trx) and NADPH this system's most general description is as a system for reducing disulfide bonds in cells. Electrons are taken from NADPH via TrxR and are transferred to the active site of Trx, which goes on to reduce protein disulfides or other substrates. The Trx system exists in all living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin
Thioredoxin (TRX or TXN) is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by ''TXN'' and ''TXN2'' genes. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the developing embryo. Although not entirely understood, thioredoxin is linked to medicine through their response to reactive oxygen species (ROS). In plants, thioredoxins regulate a spectrum of critical functions, ranging from photosynthesis to growth, flowering and the development and germination of seeds. Thioredoxins play a role in Cell signaling, cell-to-cell communication. Occurrence They are found in nearly all known organisms and are essential for life in mammals. Function The primary function of thioredoxin (Trx) is the reduction of oxidized cysteine residues and the cleavage of disulfide bonds. Multiple in vitro subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-helices
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix). The alpha helix is the most common structural arrangement in the secondary structure of proteins. It is also the most extreme type of local structure, and it is the local structure that is most easily predicted from a sequence of amino acids. The alpha helix has a right-handed helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid that is four residues earlier in the protein sequence. Other names The alpha helix is also commonly called a: * Pauling–Corey–Branson α-helix (from the names of three scientists who described its structure) * 3.613-helix because there are 3.6 amino acids in one ring, with 13 atoms being involved in the ring formed by the hydrogen bond (starting with amidic hydrogen and ending with carbonyl oxygen) Discovery In the early 1930s, William Astbury showed that there were dras ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
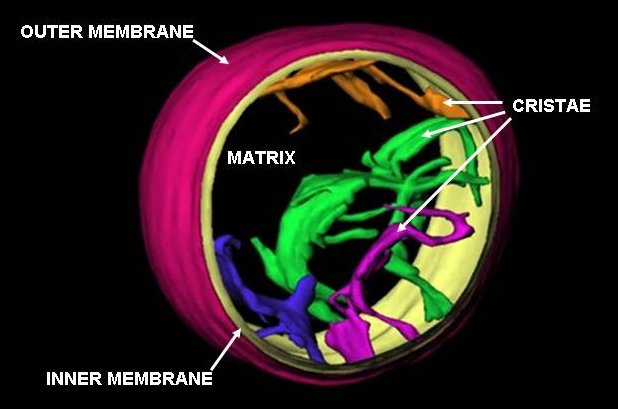
Mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal '' Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number of unicellular organisms, such as microspo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation (biochemistry), fermentation processes such as anaerobic glycolysis. The energy stored in the chemical bonds of glucose is released by the cell in the citric acid cycle, producing carbon dioxide and the energetic reducing agent, electron donors NADH and FADH. Oxidative phosphorylation uses these molecules and O2 to ATP synthase, produce ATP, which is used throughout the cell whenever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a ser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactive Oxygen Species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl radical (OH.), and singlet oxygen(1O2). ROS are pervasive because they are readily produced from O2, which is abundant. ROS are important in many ways, both beneficial and otherwise. ROS function as signals, that turn on and off biological functions. They are intermediates in the redox behavior of O2, which is central to fuel cells. ROS are central to the photodegradation of organic pollutants in the atmosphere. Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations. Inventory of ROS ROS are not uniformly defined. All sources include superoxide, singlet oxygen, and hydroxyl radical. Hydrogen peroxide is not nearly as reactive as these s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
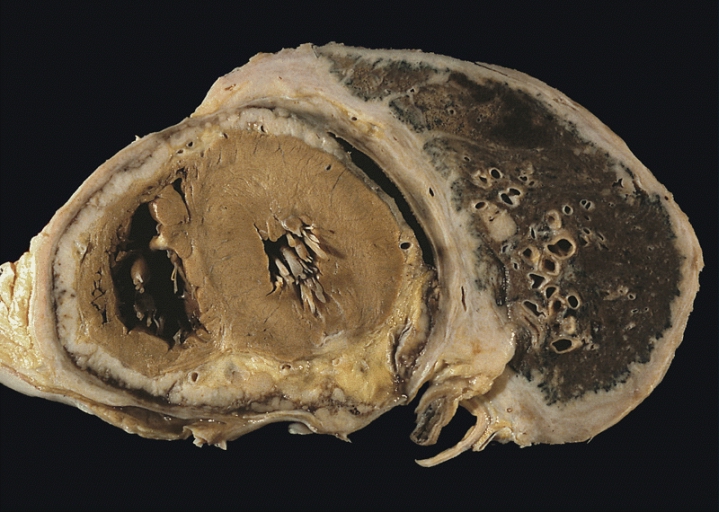
Congestive Heart Failure
Heart failure (HF), also known as congestive heart failure (CHF), is a syndrome caused by an impairment in the heart's ability to fill with and pump blood. Although symptoms vary based on which side of the heart is affected, HF typically presents with shortness of breath, excessive fatigue, and bilateral leg swelling. The severity of the heart failure is mainly decided based on ejection fraction and also measured by the severity of symptoms. Other conditions that have symptoms similar to heart failure include obesity, kidney failure, liver disease, anemia, and thyroid disease. Common causes of heart failure include coronary artery disease, heart attack, high blood pressure, atrial fibrillation, valvular heart disease, excessive alcohol consumption, infection, and cardiomyopathy. These cause heart failure by altering the structure or the function of the heart or in some cases both. There are different types of heart failure: right-sided heart failure, which affect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is a condition in which the heart becomes enlarged and cannot pump blood effectively. Symptoms vary from none to feeling tired, leg swelling, and shortness of breath. It may also result in chest pain or fainting. Complications can include heart failure, heart valve disease, or an irregular heartbeat. Causes include genetics, alcohol, cocaine, certain toxins, complications of pregnancy, and certain infections. Coronary artery disease and high blood pressure may play a role, but are not the primary cause. In many cases the cause remains unclear. It is a type of cardiomyopathy, a group of diseases that primarily affects the heart muscle. The diagnosis may be supported by an electrocardiogram, chest X-ray, or echocardiogram. In those with heart failure, treatment may include medications in the ACE inhibitor, beta blocker, and diuretic families. A low salt diet may also be helpful. In those with certain types of irregular heartbeat, blood thinner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribonucleotide Reductase
Ribonucleotide reductase (RNR), also known as ribonucleoside diphosphate reductase, is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. It catalyzes this formation by removing the 2'-hydroxyl group of the ribose ring of nucleoside diphosphates (or triphosphates depending on the class of RNR). This reduction produces deoxyribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of deoxyribonucleic acid, DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms. Furthermore, RNR plays a critical role in regulating the total rate of DNA synthesis so that DNA to cell mass is maintained at a constant ratio during cell division and DNA repair. A somewhat unusual feature of the RNR enzyme is that it catalyzes a reaction that proceeds via a free radical mechanism of action. The substrates for RNR are adenosine diphosphate, ADP, guanosine diphosphate, GDP, cytidine diphosphate, CDP and uridine diphosphate, UDP. dTDP ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Motexafin Gadolinium
Motexafin gadolinium (proposed tradename Xcytrin) is an inhibitor of thioredoxin reductase and ribonucleotide reductase. It has been proposed as a possible chemotherapeutic agent in the treatment of brain metastases. History On May 9, 2006, a New Drug Application was submitted to the United States Food and Drug Administration The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ... (FDA) by Pharmacyclics, Inc. In December 2007, the FDA issued a not approvable letter for motexafin gadolinium. References Organogadolinium compounds {{biochemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malignant Mesothelioma
Mesothelioma is a type of cancer that develops from the thin layer of tissue that covers many of the internal organs (known as the mesothelium). The area most commonly affected is the lining of the lungs and chest wall. Less commonly the lining of the abdomen and rarely the sac surrounding the heart, or the sac surrounding each testis may be affected. Signs and symptoms of mesothelioma may include shortness of breath due to fluid around the lung, a swollen abdomen, chest wall pain, cough, feeling tired, and weight loss. These symptoms typically come on slowly. More than 80% of mesothelioma cases are caused by exposure to asbestos. The greater the exposure, the greater the risk. As of 2013, about 125 million people worldwide have been exposed to asbestos at work. High rates of disease occur in people who mine asbestos, produce products from asbestos, work with asbestos products, live with asbestos workers, or work in buildings containing asbestos. Asbestos exposure and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ellman's Reagent
Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB) is a colorogenic chemical used to quantify the number or concentration of thiol groups in a sample. It was developed by George L. Ellman. Preparation In Ellman's original paper, he prepared this reagent by oxidizing 2-nitro-5-chlorobenzaldehyde to the carboxylic acid, introducing the thiol via sodium sulfide, and coupling the monomer by oxidization with iodine. Today, this reagent is readily available commercially. Ellman's test Thiols react with this compound, cleaving the disulfide bond to give 2-nitro-5-thiobenzoate (TNB−), which ionizes to the TNB2− dianion in water at neutral and alkaline pH. This TNB2− ion has a yellow color. : This reaction is rapid and stoichiometric, with the addition of one mole of thiol releasing one mole of TNB. The TNB2− is quantified in a spectrophotometer by measuring the absorbance of visible light at 412 nm, using an molar absorptivity, extinction coefficient of 14,1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |