|
Thiobacillus Ferrooxidans
''Acidithiobacillus'' is a genus of the ''Acidithiobacillia'' in the phylum "''Pseudomonadota''". This genus includes ten species of acidophilic microorganisms capable of sulfur and/or iron oxidation: ''Acidithiobacillus albertensis, Acidithiobacillus caldus, Acidithiobacillus cuprithermicus, Acidithiobacillus ferrianus, Acidithiobacillus ferridurans, Acidithiobacillus ferriphilus, Acidithiobacillus ferrivorans, Acidithiobacillus ferrooxidans, Acidithiobacillus sulfuriphilus,'' and ''Acidithiobacillus thiooxidans.'' ''A. ferooxidans'' is the most widely studied of the genus, but ''A. caldus'' and ''A. thiooxidans'' are also significant in research. Like all ''"Pseudomonadota"'', ''Acidithiobacillus'' spp. are Gram-negative and non-spore forming. They also play a significant role in the generation of acid mine drainage; a major global environmental challenge within the mining industry. Some species of ''Acidithiobacillus'' are utilized in bioleaching and biomining. A portion of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horizontal Gene Transfer
Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). HGT is an important factor in the evolution of many organisms. HGT is influencing scientific understanding of higher-order evolution while more significantly shifting perspectives on bacterial evolution. Horizontal gene transfer is the primary mechanism for the spread of antibiotic resistance in bacteria, and plays an important role in the evolution of bacteria that can degrade novel compounds such as human-created Bactericide, pesticides and in the evolution, maintenance, and transmission of virulence. It often involves Temperateness (virology), temperate bacteriophages and plasmids. Genes responsible for antibiotic resistance in one species of bacteria can be transferred to another species of bacteria through various mechanisms of HGT such as Transformation (genetics), tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biogenic Sulfide Corrosion
Biogenic sulfide corrosion is a bacterially mediated process of forming hydrogen sulfide gas and the subsequent conversion to sulfuric acid that attacks concrete and steel within wastewater environments. The hydrogen sulfide gas is biochemically oxidized in the presence of moisture to form sulfuric acid. The effect of sulfuric acid on concrete and steel surfaces exposed to severe wastewater environments can be devastating. In the USA alone, corrosion causes sewer asset losses estimated at $14 billion per year. This cost is expected to increase as the aging infrastructure continues to fail. Environment Corrosion may occur where stale sewage generates hydrogen sulfide gas into an atmosphere containing oxygen gas and high relative humidity. There must be an underlying anaerobic aquatic habitat containing sulfates and an overlying aerobic aquatic habitat separated by a gas phase containing both oxygen and hydrogen sulfide at concentrations in excess of 2 ppm.Sawyer&McCarty p.461&462 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric
In chemistry, iron(III) or ''ferric'' refers to the chemical element, element iron in its +3 oxidation number, oxidation state. ''Ferric chloride'' is an alternative name for iron(III) chloride (). The adjective ''ferrous'' is used instead for iron(II) salts, containing the cation Fe2+. The word ''wikt:ferric, ferric'' is derived from the Latin word , meaning "iron". Although often abbreviated as Fe3+, that naked ion does not exist except under extreme conditions. Iron(III) centres are found in many compounds and coordination complexes, where Fe(III) is bonded to several Ligand, ligands. A molecular ferric complex is the anion ferrioxalate, , with three bidentate oxalate ions surrounding the Fe core. Relative to lower oxidation states, ferric is less common in organoiron chemistry, but the ferrocenium cation is well known. Iron(III) in biology All known forms of life require iron, which usually exists in Fe(II) or Fe(III) oxidation states. Many proteins in living beings cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
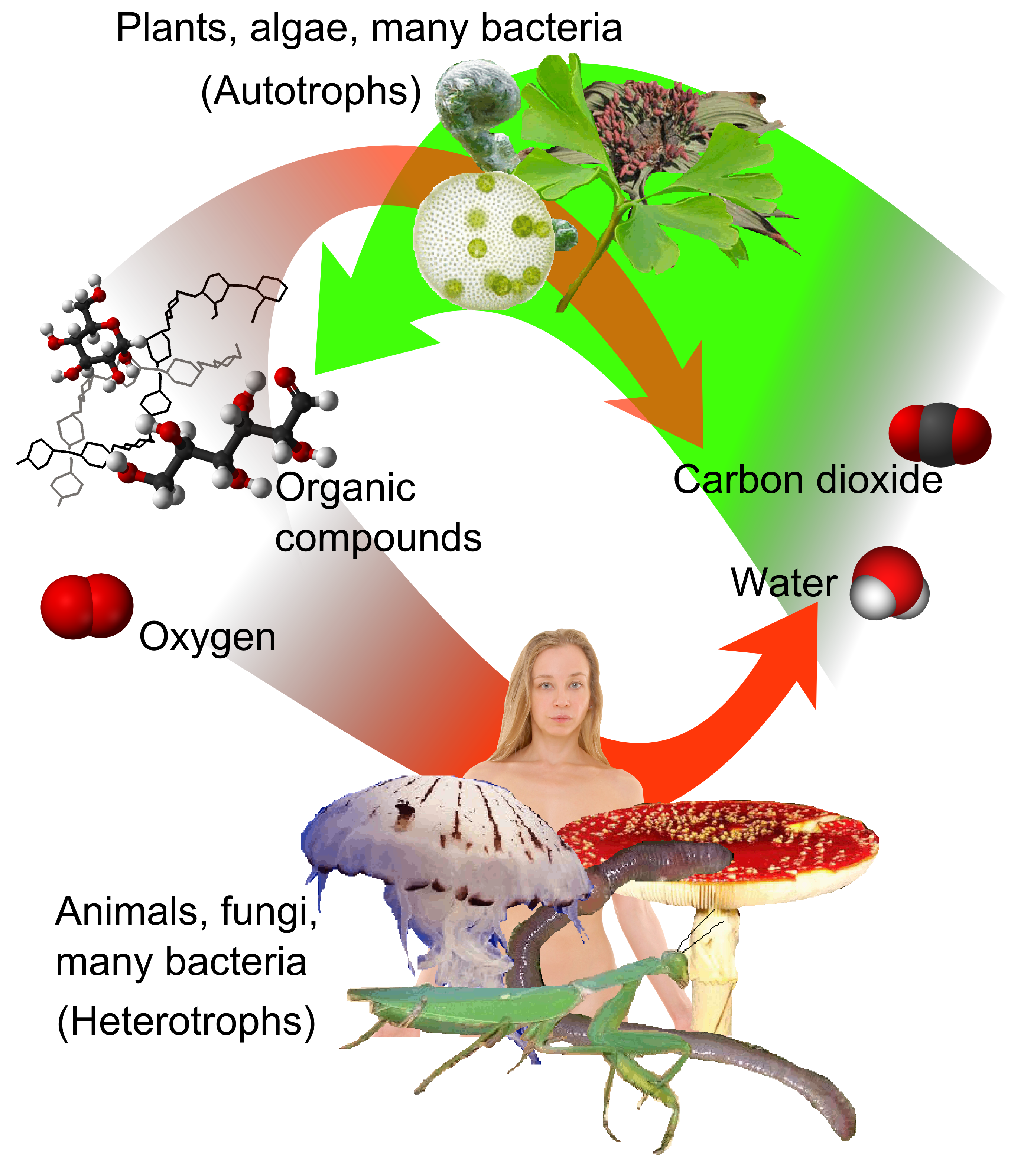
Autotrophic
An autotroph is an organism that can convert abiotic sources of energy into energy stored in organic compounds, which can be used by other organisms. Autotrophs produce complex organic compounds (such as carbohydrates, fats, and proteins) using carbon from simple substances such as carbon dioxide,Morris, J. et al. (2019). "Biology: How Life Works", 3rd edition, W. H. Freeman. generally using energy from light or inorganic chemical reactions. Autotrophs do not need a living source of carbon or energy and are the producers in a food chain, such as plants on land or algae in water. Autotrophs can reduce carbon dioxide to make organic compounds for biosynthesis and as stored chemical fuel. Most autotrophs use water as the reducing agent, but some can use other hydrogen compounds such as hydrogen sulfide. The primary producers can convert the energy in the light ( phototroph and photoautotroph) or the energy in inorganic chemical compounds (chemotrophs or chemolithotrophs) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrite
The mineral pyrite ( ), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral. Pyrite's metallic luster and pale brass-yellow hue give it a superficial resemblance to gold, hence the well-known nickname of ''fool's gold''. The color has also led to the nicknames ''brass'', ''brazzle'', and ''brazil'', primarily used to refer to pyrite found in coal. The name ''pyrite'' is derived from the Greek (), 'stone or mineral which strikes fire', in turn from (), 'fire'. In ancient Roman times, this name was applied to several types of stone that would create sparks when struck against steel; Pliny the Elder described one of them as being brassy, almost certainly a reference to what is now called pyrite. By Georgius Agricola's time, , the term had become a generic term for all of the sulfide minerals. Pyrite is usually found associated with other sulfides or oxides in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiobacillus
''Thiobacillus'' is a genus of Gram-negative Betaproteobacteria. ''Thiobacillus thioparus'' is the type species of the genus, and the type strain thereof is the StarkeyT strain, isolated by Robert Starkey in the 1930s from a field at Rutgers University in the United States of America. While over 30 "species" have been named in this genus since it was defined by Martinus Beijerinck in 1904, (the first strain was observed by the biological oceanographer Alexander Nathansohn in 1902 - likely what we would now call '' Halothiobacillus neapolitanus''), most names were never validly or effectively published. The remainder were either reclassified into '' Paracoccus'', '' Starkeya'' (both in the Alphaproteobacteria); ''Sulfuriferula'', '' Annwoodia'', '' Thiomonas'' (in the Betaproteobacteria); '' Halothiobacillus'', '' Guyparkeria'' (in the Gammaproteobacteria), or ''Thermithiobacillus'' or ''Acidithiobacillus'' (in the Acidithiobacillia). The very loosely defined "species" ''Thio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acidithiobacillales
The ''Acidithiobacillales'' are an order of bacteria within the class '' Acidithiobacillia'' and comprises the genera ''Acidithiobacillus'' and ''Thermithiobacillus''. Originally, both were included in the genus '' Thiobacillus'', but they are not related to the type species, which belongs to the '' Betaproteobacteria''.Kelly (D.P.) and Wood (A.P.): ''Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov.'' In: ''International Journal of Systematic and Evolutionary Microbiology, 2000, 50, 511-516.'' References External linksAcidithiobacillalesLPSN List of Prokaryotic names with Standing in Nomenclature Acidithiobacillia Acidophiles {{gammaproteobacteria-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calvin-Benson-Bassham Cycle
The Calvin cycle, light-independent reactions, bio synthetic phase, dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products ( ATP and NADPH) of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and the reducing power of NADPH from the light-dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation (redox) reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of to a sugar. There are three phases to the ligh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |