|
Thermocompression Bonding
Thermocompression bonding describes a wafer bonding technique and is also referred to as diffusion bonding, pressure joining, thermocompression welding or solid-state welding. Two metals, e.g. gold- gold (Au), are brought into atomic contact applying force and heat simultaneously. The diffusion requires atomic contact between the surfaces due to the atomic motion. The atoms migrate from one crystal lattice to the other one based on crystal lattice vibration. This atomic interaction sticks the interface together. The diffusion process is described by the following three processes: * surface diffusion * grain boundary diffusion * bulk diffusion This method enables internal structure protecting device packages and direct electrical interconnect structures without additional steps beside the surface mounting process. Overview The most established materials for thermocompression bonding are copper (Cu), gold (Au) and aluminium (Al) because of their high diffusion rates. In addit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wafer Bonding
Wafer bonding is a packaging technology on wafer-level for the fabrication of microelectromechanical systems (MEMS), nanoelectromechanical systems (NEMS), microelectronics Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre ... and optoelectronics, ensuring a mechanically stable and hermetically sealed encapsulation. The wafers' diameter range from 100 mm to 200 mm (4 inch to 8 inch) for MEMS/NEMS and up to 300 mm (12 inch) for the production of microelectronic devices. Smaller wafers were used in the early days of the microelectronics industry, with wafers being just 1 inch in diameter in the 1950s. Overview In microelectromechanical systems (MEMS) and nanoelectromechanical systems (NEMS), the package protects the sensitive internal structures from environmental infl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of (called a passivation layer) that protects the foil from further oxidation.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry Oxides are extraordinarily diverse in terms of stoichiometries (the measurable relationship between reactants and chemical equations of an equation or reaction) and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnsh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate unt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma (physics)
Plasma () is a state of matter characterized by the presence of a significant portion of charged particles in any combination of ions or electrons. It is the most abundant form of ordinary matter in the universe, mostly in stars (including the Sun), but also dominating the rarefied intracluster medium and Outer space#Intergalactic space, intergalactic medium. Plasma can be artificially generated, for example, by heating a neutral gas or subjecting it to a strong electromagnetic field. The presence of charged particles makes plasma electrically conductive, with the dynamics of individual particles and macroscopic plasma motion governed by collective electromagnetic fields and very sensitive to externally applied fields. The response of plasma to electromagnetic fields is used in many modern devices and technologies, such as plasma display, plasma televisions or plasma etching. Depending on temperature and density, a certain number of neutral particles may also be present, in wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
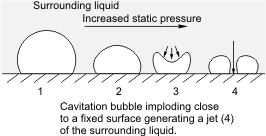
Cavitation
Cavitation in fluid mechanics and engineering normally is the phenomenon in which the static pressure of a liquid reduces to below the liquid's vapor pressure, leading to the formation of small vapor-filled cavities in the liquid. When subjected to higher pressure, these cavities, called "bubbles" or "voids", collapse and can generate shock waves that may damage machinery. These shock waves are strong when they are very close to the imploded bubble, but rapidly weaken as they propagate away from the implosion. Cavitation is a significant cause of wear in some engineering contexts. Collapsing voids that implode near to a metal surface cause cyclic stress through repeated implosion. This results in surface fatigue of the metal, causing a type of wear also called "cavitation". The most common examples of this kind of wear are to pump impellers, and bends where a sudden change in the direction of liquid occurs. Cavitation is usually divided into two classes of behavior. ''In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passivation (chemistry)
In physical chemistry and engineering, passivation is coating a material so that it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. Undesired passivation of electrodes, called "fouling", increases the circuit resistance so it interferes with some electrochemical applications such as electrocoagulation for wastewater treatment, amperometric chemical sensing, and electrochemical synthesis. When exposed to air, many metals naturally form a hard, relatively inert surface layer, usually an oxide (termed the "native oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Etching
Dry etching refers to the removal of material, typically a masked pattern of semiconductor material, by exposing the material to a bombardment of ions (usually a plasma of reactive gases such as fluorocarbons, oxygen, chlorine, boron trichloride; sometimes with addition of nitrogen, argon, helium and other gases) that dislodge portions of the material from the exposed surface. A common type of dry etching is reactive-ion etching. Unlike with many (but not all, see isotropic etching) of the wet chemical etchants used in wet etching, the dry etching process typically etches directionally or anisotropically. Applications Dry etching is used in conjunction with photolithographic techniques to attack certain areas of a semiconductor surface in order to form recesses in material. Applications include contact holes (which are contacts to the underlying semiconductor substrate), via holes (which are holes that are formed to provide an interconnect path between conductive layers in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etching (microfabrication)
Etching is used in microfabrication to chemically remove layers from the surface of a wafer (electronics), wafer during manufacturing. Etching is a critically important process module in fabrication, and every wafer undergoes many etching steps before it is complete. For many etch steps, part of the wafer is protected from the etchant by a "masking" material which resists etching. In some cases, the masking material is a photoresist which has been patterned using photolithography. Other situations require a more durable mask, such as silicon nitride. Etching media and technology The two fundamental types of etchants are liquid-phase ("wet") and plasma (physics), plasma-phase ("dry"). Each of these exists in several varieties. Wet etching The first etching processes used liquid-phase ("wet") etchants. This process is now largely outdated but was used up until the late 1980s when it was superseded by dry plasma etching. The wafer can be immersed in a bath of etchant, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Impurity
In chemistry and materials science, impurities are chemical substances inside a confined amount of liquid, gas, or solid. They differ from the chemical composition of the material or compound. Firstly, a pure chemical should appear in at least one Phase (matter), chemical phase and can also be characterized by its phase diagram. Secondly, a pure chemical should prove to be homogeneous (i.e., a uniform substance that has the same composition throughout the material). The perfect pure chemical will pass all attempts to separate and purify it further. Thirdly, and here we focus on the common chemical definition, it should not contain any trace of any other kind of chemical species. In reality, there are no absolutely 100% pure chemical compounds, as there is always some small amount of contamination. The levels of impurities in a material are generally defined in relative terms. standardization, Standards have been established by various organizations that attempt to define the permi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coefficient Of Thermal Expansion
Thermal expansion is the tendency of matter to increase in length, area, or volume, changing its size and density, in response to an increase in temperature (usually excluding phase transitions). Substances usually contract with decreasing temperature (thermal contraction), with rare exceptions within limited temperature ranges ('' negative thermal expansion''). Temperature is a monotonic function of the average molecular kinetic energy of a substance. As energy in particles increases, they start moving faster and faster, weakening the intermolecular forces between them and therefore expanding the substance. When a substance is heated, molecules begin to vibrate and move more, usually creating more distance between themselves. The relative expansion (also called strain) divided by the change in temperature is called the material's coefficient of linear thermal expansion and generally varies with temperature. Prediction If an equation of state is available, it can be used t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |