|
Tetramethylstannane
Tetramethyltin is an organometallic compound with the formula (CH3)4Sn. This liquid, one of the simplest organotin compounds, is useful for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. It is volatile and toxic, so care should be taken when using it in the laboratory. Synthesis and structure Tetramethyltin is synthesized by reaction of the Grignard reagent methylmagnesium iodide, with tin tetrachloride, which is synthesized by reacting tin metal with chlorine gas. :4 CH3MgI + SnCl4 → (CH3)4Sn + 4 MgICl In tetramethyltin, the metal surrounded by four methyl groups in a tetrahedral structure is a heavy analogue of neopentane. Applications Precursor to methyltin compounds Tetramethyltin is a precursor to trimethyltin chloride (and related methyltin halides), which are precursors to other organotin compounds. These methyltin chlorides are prepared via the so-called Kocheshkov redistribution reaction. Thus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neopentane
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure. Neopentane is the simplest alkane with a quaternary carbon, and has achiral tetrahedral symmetry. It is one of the three structural isomers with the molecular formula C5H12 (pentanes), the other two being ''n''-pentane and isopentane. Out of these three, it is the only one to be a gas at standard conditions; the others are liquids. It was first synthesized by Russian chemist in 1870. Nomenclature The traditional name neopentane, coined by William Odling in 1876, was still retained in the 1993 IUPAC recommendations, but is no longer recommended according to the 2013 recommendations. The preferred IUPAC name is the systematic name 2,2-dimethylpropane, but the substituent numbers are su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraethyltin
Tetraethyltin or tetraethyl tin is a chemical compound with the formula , that is, a tin atom attached to four ethyl groups. It is an important example of an organotin compound, often abbreviated as TET. Tetraethyltin is a colourless flammable liquid, soluble in diethyl ether and insoluble in water, that freezes at −112 °C and boils at 181 °C.SAFC corp''tetraethyltin''catalog page. Accessed on 2011-01-18. It is used in the electronics industry. Tetraethyltin can be obtained by reacting ethylmagnesium bromide with tin(IV) chloride:G. J. M. Van Der Kerk and J. G. A. Luijten (1956)"Tetraethyltin" ''Organic Syntheses'', volume 36, page 86; Coll. Vol. 4, p.881 (1963) : The same reaction can be used to obtain tetra-''n''-propyltin and tetra-''n''-butyltin. Tetraethyltin is converted in the body to the more toxic triethylstannylium ions.Jill E. Cremer (1958), "The biochemistry of organotin compounds. The conversion of tetraethyltin into triethyltin in mammals". ''Bioc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are arccos(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group ''Td'', but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram at left, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compounds
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known, but are less imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
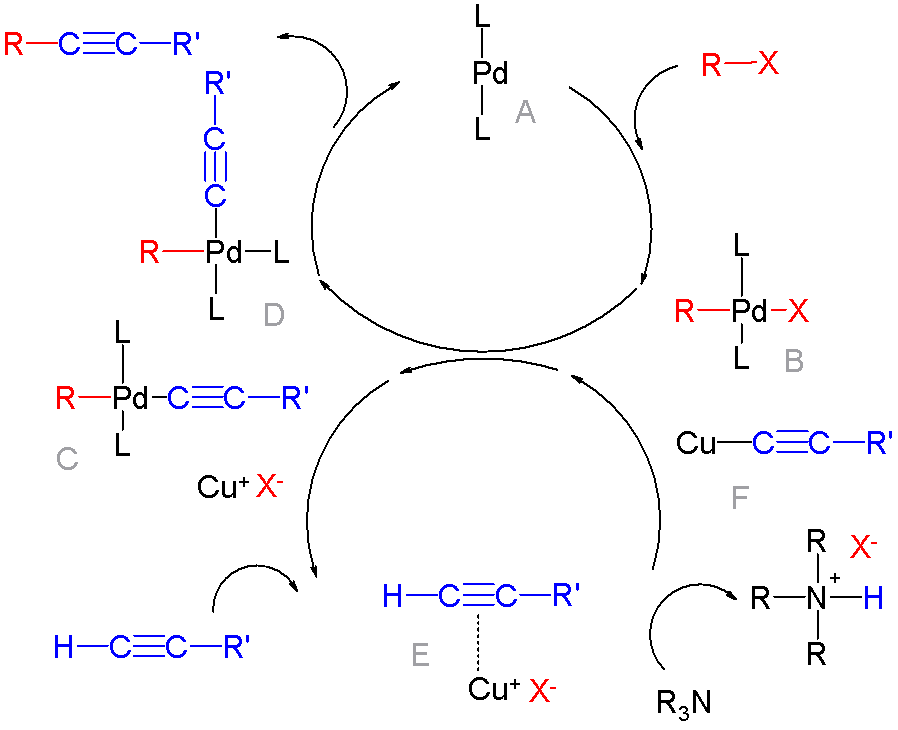
Palladium-catalyzed Coupling Reactions
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reaction type is this: : (R, R' = organic fragments, usually aryl; M = main group center such as Li or MgX; X = halide) These reactions are used to form carbon–carbon bonds but also carbon-heteroatom bonds. Cross-coupling reaction are a subset of coupling reactions. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism Many mechanisms exist reflecting the myriad types of cross-couplings, including those that do not require metal catalysts. Often, however, cross-coupling refers to a metal-catalyzed reaction of a nucleophilic partner with an electrophilic partner. In such cases, the mechanism generally involves reductive elimin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, Enantioselective synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject. Total synthesis A total synthesis refers to the complete chemical synthesis of molecules from simple, Precursor (chemistry), natural precursors. Total synthesis is accomplished either via a linear or convergent approach. In a Linear synthesis, ''linear'' synthesis—often adequate for simple structures—several steps are performed sequentially until the molecule is com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeolite
Zeolites are a group of several microporous, crystalline aluminosilicate minerals commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. The term was originally coined in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heating a material, believed to have been stilbite, produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material ''zeolite'', from the Greek , meaning "to boil" and , meaning "stone". Zeolites occur naturally, but are also produced industrially on a large scale. , 253 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained is examined by the International Zeolite Association Structure Commission (IZA-SC) and receives a three-letter designation. Character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows Tetrahedral molecular geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyvinylchloride
Polyvinyl chloride (alternatively: poly(vinyl chloride), colloquial: vinyl or polyvinyl; abbreviated: PVC) is the world's third-most widely produced synthetic polymer of plastic (after polyethylene and polypropylene). About 40 million tons of PVC are produced each year. PVC comes in rigid (sometimes abbreviated as RPVC) and flexible forms. Rigid PVC is used in construction for pipes, doors and windows. It is also used in making plastic bottles, packaging, and bank or membership cards. Adding plasticizers makes PVC softer and more flexible. It is used in plumbing, electrical cable insulation, flooring, signage, phonograph records, inflatable products, and in rubber substitutes. With cotton or linen, it is used in the production of canvas. Polyvinyl chloride is a white, brittle solid. It is soluble in ketones, chlorinated solvents, dimethylformamide, THF and DMAc. Discovery PVC was synthesized in 1872 by German chemist Eugen Baumann after extended investigation and experime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Chloride
Mercury(II) chloride (mercury bichloride, mercury dichloride, mercuric chloride), historically also sulema or corrosive sublimate, is the inorganic chemical compound of mercury and chlorine with the formula HgCl2, used as a laboratory reagent. It is a white crystalline solid and a molecular compound that is very toxic to humans. Once used as a first line treatment for syphilis, it has been replaced by the more effective and less toxic procaine penicillin since at least 1948. Synthesis Mercuric chloride is obtained by the action of chlorine on mercury or on mercury(I) chloride. It can also be produced by the addition of hydrochloric acid to a hot, concentrated solution of mercury(I) compounds such as the nitrate: :Hg2(NO3)2 + 4 HCl → 2 HgCl2 + 2 H2O + 2 NO2 Heating a mixture of solid mercury(II) sulfate and sodium chloride also affords volatile HgCl2, which can be separated by sublimation. Properties Mercuric chloride is not a salt composed of discrete ions, but it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethyltin Chloride
Trimethyltin chloride is an organotin compound with the formula . It is a white solid that is highly toxic and malodorous. It is susceptible to hydrolysis. Synthesis Trimethyltin chloride can be prepared by the Redistribution (chemistry), redistribution reaction of tetramethyltin with tin tetrachloride. : This redistribution reaction is typically performed with no solvent because high temperatures are required and purification is simplified. A second route to involves treating the corresponding Organotin chemistry#Organotin oxides and hydroxides, hydroxide or oxide (in the following reaction, trimethyltin hydroxide ) with a halogenating agent such as hydrogen chloride or thionyl chloride (): : Uses Trimethyltin chloride is used as a source of the trimethylstannyl group (). For example, it is a precursor to vinyltrimethylstannane () and indene, indenyltrimethylstanane (see Transition metal indenyl complex): : : An example of an Organolithium reagent, organolithium reagent reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |