|
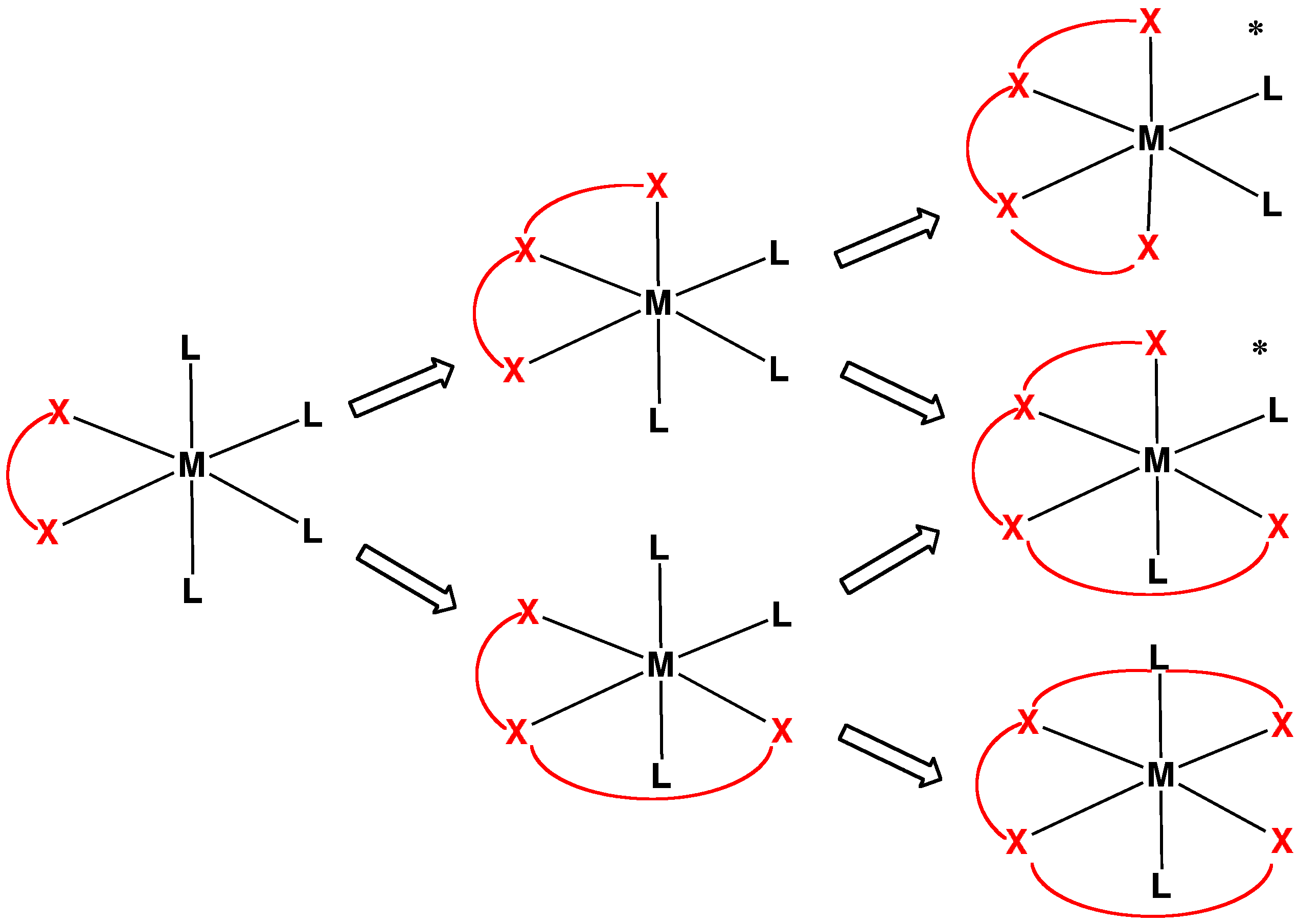
Tetradentate
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands. Tetradentate ligands are common in nature in the form of chlorophyll, which has a core ligand called chlorin, and heme, which has a core ligand called porphyrin. They are responsible for the colour observed in plants and humans. Phthalocyanine is an artificial macrocyclic tetradentate ligand that is used to make blue and green pigments. Shape Tetradentate ligands can be classified by the topology of the connections between donor atoms. Common forms are linear (also called sequential), ring or tripodal. A tetrapodal ligand that is also tetradentate has four legs with donor atoms and a bridgehead that is not a donor. Upon binding with a central atom, there are several arrangements possible (known as geometric isomers). Linear ligands A linear tetradentate ligand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylenediaminediacetic Acid
Ethylenediaminediacetic acid (EDDA) is the organic compound with the formula C2H4(NHCH2CO2H)2. It is a derivative of two molecules of glycine, wherein the amines are linked. It is a white solid. It is one of several aminopolycarboxylic acids. The conjugate base is a tetradentate ligand. A representative complex is Na o(EDDA)(CO3){{cite book , volume=18 , author=Leon J. Halloran , author2=Arlene L. Gillie , author3=J. Ivan Legg , title=Inorganic Syntheses , chapter=Ethylenediamine- ''N,N'' ′-Diacetic Acid Complexes of Cobalt(III) , pages=103–111, year=1978, doi=10.1002/9780470132494.ch17, isbn=978-0-470-13249-4 Related compounds *ethylenediaminetetraacetic acid Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula . This white, slightly water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-solubl ... References Chelating agents Dicarboxylic acids Tetradentate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C2-Symmetric Ligands
In homogeneous catalysis, ''C''2-symmetric ligands refer to ligands that lack mirror symmetry but have ''C''2 symmetry (two-fold rotational symmetry). Such ligands are usually bidentate and are valuable in catalysis. The ''C''2 symmetry of ligands limits the number of possible reaction pathways and thereby increases enantioselectivity, relative to asymmetrical analogues. ''C''2-symmetric ligands are a subset of chiral ligands. Chiral ligands, including ''C''2-symmetric ligands, combine with metals or other groups to form chiral catalysts. These catalysts engage in enantioselective chemical synthesis, in which chirality in the catalyst yields chirality in the reaction product. Examples An early ''C''2-symmetric ligand, diphosphine catalytic ligand DIPAMP, was developed in 1968 by William S. Knowles and coworkers of Monsanto Company, who shared the 2001 Nobel Prize in Chemistry. This ligand was used in the industrial production of -DOPA. : Some classes of ''C''2-symmetric li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trigonal Bipyramid
A triangular bipyramid is a hexahedron with six triangular faces constructed by attaching two tetrahedra face-to-face. The same shape is also known as a triangular dipyramid or trigonal bipyramid. If these tetrahedra are regular, all faces of a triangular bipyramid are equilateral. It is an example of a deltahedron, composite polyhedron, and Johnson solid. Many polyhedra are related to the triangular bipyramid, such as similar shapes derived from different approaches and the triangular prism as its dual polyhedron. Applications of a triangular bipyramid include trigonal bipyramidal molecular geometry which describes its atom cluster, a solution of the Thomson problem, and the representation of color order systems by the eighteenth century. Special cases As a right bipyramid Like other bipyramids, a triangular bipyramid can be constructed by attaching two tetrahedra face-to-face. These tetrahedra cover their triangular base, and the resulting polyhedron has six triangles, fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclen
Cyclen (1,4,7,10-tetraazacyclododecane) is an aza-crown ether with the formula (CH2CH2NH)4. It is a white solid. It forms Coordination complex, coordination complexes with metal cations and is used to synthesize the chelating agent DOTA (chelator), DOTA which has several medical applications. Being structurally simple, symmetrical, and polyfunctional, cyclen has been widely investigated. Synthesis Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates: :TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4 The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine. High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a amidine, bisamidine – also a bis(2-Imidazoline, imidazoline) – followed by organic reduction, reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclam
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transition metal cations. The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine. The compound features four secondary amines. Its complexes therefore can exist as several diastereomers, depending on the relative orientation of the N–H centres. Its complexes feature alternating five- and six-membered chelate rings. The closely related ligand cyclen ((CH2CH2NH)4) forms only five-membered C2N2M chelate rings and tends not to form square-planar complexes. ''N''-Alkyl derivatives Metal-cyclam complexes are prone to oxidative degradation, which is initiated by deprotonation of the secondary amine. This flaw led to the development of cyclam derivatives wherein the NH centres are replaced by tertiary amines. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. They are lustrous metals with good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramagnetic because of their unpaired d electrons, as are many of their compounds. All of the elements that are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclam
Cyclam (1,4,8,11-tetraazacyclotetradecane) is an organic compound with the formula (NHCH2CH2NHCH2CH2CH2)2. Classified as an aza-crown ether, it is a white solid that is soluble in water. As a macrocyclic ligand, it binds strongly to many transition metal cations. The compound was first prepared by the reaction of 1,3-dibromopropane and ethylenediamine. The compound features four secondary amines. Its complexes therefore can exist as several diastereomers, depending on the relative orientation of the N–H centres. Its complexes feature alternating five- and six-membered chelate rings. The closely related ligand cyclen ((CH2CH2NH)4) forms only five-membered C2N2M chelate rings and tends not to form square-planar complexes. ''N''-Alkyl derivatives Metal-cyclam complexes are prone to oxidative degradation, which is initiated by deprotonation of the secondary amine. This flaw led to the development of cyclam derivatives wherein the NH centres are replaced by tertiary amines. F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
12-crown-4 Skeletal
1 (one, unit, unity) is a number, numeral, and glyph. It is the first and smallest positive integer of the infinite sequence of natural numbers. This fundamental property has led to its unique uses in other fields, ranging from science to sports, where it commonly denotes the first, leading, or top thing in a group. 1 is the unit of counting or measurement, a determiner for singular nouns, and a gender-neutral pronoun. Historically, the representation of 1 evolved from ancient Sumerian and Babylonian symbols to the modern Arabic numeral. In mathematics, 1 is the multiplicative identity, meaning that any number multiplied by 1 equals the same number. 1 is by convention not considered a prime number. In digital technology, 1 represents the "on" state in binary code, the foundation of computing. Philosophically, 1 symbolizes the ultimate reality or source of existence in various traditions. In mathematics The number 1 is the first natural number after 0. Each natural number, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrin
Corrin is a heterocyclic compound. Although not known to exist on its own, the molecule is of interest as the parent macrocycle related to the cofactor and chromophore in vitamin B12. Its name reflects that it is the "core" of vitamin B12 (cobalamins). Compounds with a corrin core are known as "corrins". There are two chiral centres, which in natural compounds like cobalamin have the same stereochemistry. Coordination chemistry Upon deprotonation, the corrinoid ring is capable of binding cobalt. In vitamin B12, the resulting complex also features a benzimidazole-derived ligand, and the sixth site on the octahedron serves as the catalytic center. The corrin ring resembles the porphyrin ring. Both feature four pyrrole-like subunits organized into rings. Corrins have a central 15-membered ring whereas porphryins have an interior 16-membered ring. All four nitrogen centers are linked by conjugation structure, with alternating double and single bonds. In contrast to po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |