|
Tetraborane
Tetraborane (systematically named ''arachno''-tetraborane(10)) was the first boron hydride compound to be discovered. It was classified by Alfred Stock and Carl Massenez in 1912 and was first isolated by Stock. It has a relatively low boiling point at 18 °C and is a gas at room temperature. Tetraborane gas is foul smelling and toxic. History The class of boranes was elucidated using X-ray diffraction analysis by Lipscomb et al. in the 1950s. The X-ray data indicated two-electron multicenter bonds. Later, analysis based on high-resolution X-ray data was performed to analyze the charge density. Structure Like other boron hydride clusters, the structure of tetraborane involves multicenter bonding, with hydrogen bridges or protonated double bonds. According to its formula, B4H10, it is classified as an ''arachno''-cluster and has a butterfly geometry, which can be rationalized by Wade's rules. Each boron is ''sp3'' hybridized, and “the configuration of the three hydrogens surr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
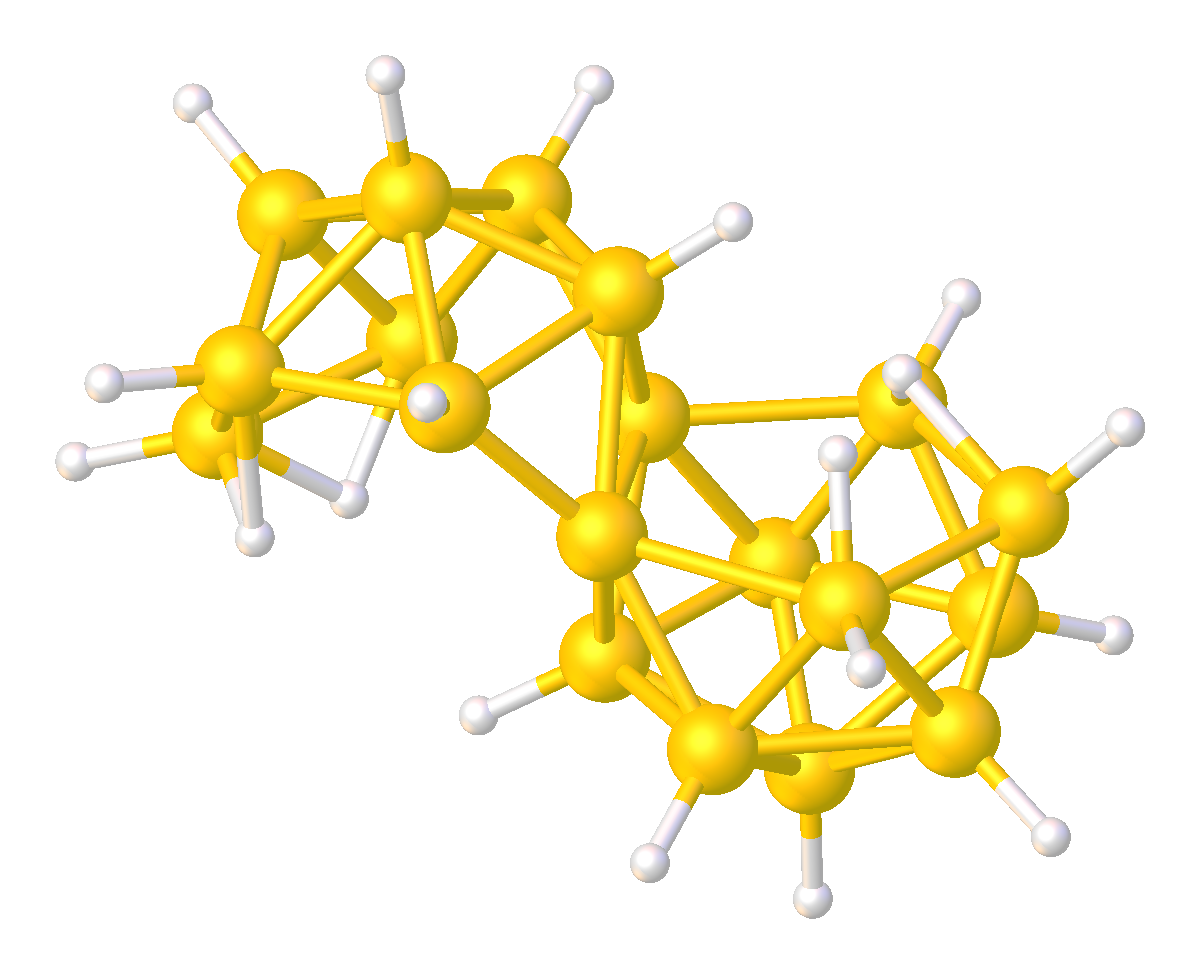
Boron Hydride Clusters
Boron hydride clusters are compounds with the formula or related anions, where x ≥ 3. Many such cluster compounds are known. Common examples are those with 5, 10, and 12 boron atoms. Although they have few practical applications, the borane hydride clusters exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed. History The development of the borane hydride clusters resulted from pioneering work by Alfred Stock, invented the glass vacuum line for their study. The structures of the boron hydride clusters were determined beginning in 1948 with the characterization of decaborane. William Lipscomb was awarded the Nobel Prize in Chemistry in 1976 for this and many subsequent crystallographic investigations. These investigations revealed the prevalence of deltahedral structures, i.e., networks of triangular arrays of BH centers. The bonding of the clusters ushered in P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boranes
A borane is a compound with the formula although examples include multi-boron derivatives. A large family of boron hydride clusters is also known. In addition to some applications in organic chemistry, the boranes have attracted much attention as they exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes, are also a well developed class of compounds. pp 151-195 History The development of the chemistry of boranes led to innovations in synthetic methods as well as structure and bonding. First, new synthetic techniques were required to handle diborane and many of its derivatives, which are both pyrophoric and volatile. Alfred Stock invented the glass vacuum line for this purpose. The structure of diborane was correctly predicted in 1943 many years after its discovery. Interest in boranes increased during World War II due to the potential of uranium borohydride for enrichment of the uranium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wurtz Reaction
In organic chemistry, the Wurtz reaction, named after Charles Adolphe Wurtz, is a coupling reaction in which two alkyl halides are treated with sodium metal to form a higher alkane. : 2 R−X + 2 Na → R−R + 2 NaX The reaction is of little value because yields are low. Exceptions are some intramolecular versions, such as 1,6-dibromohexane + 2 Na → cyclohexane + 2 NaBr. A related reaction, which combines alkyl halides with aryl halides is called the Wurtz–Fittig reaction. Despite its very modest utility, the Wurtz reaction is widely cited as representative of reductive coupling. Mechanism The reaction proceeds by an initial metal–halogen exchange, which is described with the following idealized stoichiometry: : R−X + 2 M → RM + MX This step may involve the intermediacy of radical species R·. The conversion resembles the formation of a Grignard reagent. The RM intermediates have been isolated in several cases. The radical is susceptible to diverse reactions. The or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and dry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diborane
Diborane(6), commonly known as diborane, is the chemical compound with the formula . It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents. Structure and bonding The structure of diborane has D2h symmetry. Four hydrides are terminal, while two bridge between the boron centers. The lengths of the B–Hbridge bonds and the B–Hterminal bonds are 1.33 and 1.19 Å respectively. This difference in bond lengths reflects the difference in their strengths, the B–Hbridge bonds being relatively weaker. The weakness of the B–Hbridge compared to B–Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ≈2100 and 2500 cm−1 respectively. The model determined by molecular orbital theory describes the bonds between boron and the te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology The word ''pyrolysis'' is coined from the Greek language, Greek-derived morpheme, elements ''pyro-'' (from Ancient Greek : - "fire, heat, fever") and ''lysis'' ( : - "separation, loosening"). Applications Pyrolysis is most commonly used in the treatment of organic compound, organic materials. It is one of the processes involved in the charring of wood or pyrolysis of biomass. In general, pyrolysis of organic substances produces volatile products and leaves Char (chemistry), char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered one of the steps in the processes of gasification or combustion. Laypeople often confuse pyrolysis gas with syngas. Pyrolysis gas has a high percentage of heavy tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfred Stock
Alfred Stock (July 16, 1876 – August 12, 1946) was a German inorganic chemist. He did pioneering research on the hydrides of boron and silicon, coordination chemistry, mercury, and mercury poisoning. The German Chemical Society's Alfred-Stock Memorial Prize is named after him. Life Stock was born in Danzig (Gdańsk) and educated at the Friedrich-Werder grammar school in Berlin. In 1894 he began studies in chemistry at the Friedrich Wilhelm-University in Berlin. After finishing his dissertation about the quantitative separation of arsenic and antimony in the works of Emil Fischer he got his doctoral degree. In 1899 he worked with the French chemist and toxicologist Henri Moissan in Paris for one year. He was given the task of synthesizing still unknown compounds of boron and silicon. Five years later he became professor at the University of Breslau. In 1916 he succeeded Richard Willstätter as director at the Kaiser Wilhelm Institute for Chemistry in Berlin. After a sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |