|
THBD
Thrombomodulin (TM), CD141 or BDCA-3 is an integral membrane protein expressed on the surface of endothelial cells and serves as a cofactor for thrombin. It reduces blood coagulation by converting thrombin to an anticoagulant enzyme from a procoagulant enzyme. Thrombomodulin is also expressed on human mesothelial cell, monocyte and a dendritic cell subset. Genetics and structure In humans, thrombomodulin is encoded by the gene. The protein has a molecular mass of 74k Da, and consists of a single chain with six tandemly repeated EGF-like domains, a Serine/Threonine-rich spacer and a transmembrane domain. It is a member of the C-type lectin domain (CTLD) group 14 family. Function Thrombomodulin functions as a cofactor in the thrombin-induced activation of protein C in the anticoagulant pathway by forming a 1:1 stoichiometric complex with thrombin. This raises the speed of protein C activation thousandfold. Thrombomodulin-bound thrombin has procoagulant effect at the sam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin Domain
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EGF-like Domain
The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Of Differentiation
The cluster of differentiation (also known as cluster of designation or classification determinant and often abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules providing targets for immunophenotyping of cells. In terms of physiology, CD molecules can act in numerous ways, often acting as receptors or ligands important to the cell. A signal cascade is usually initiated, altering the behavior of the cell (see cell signaling). Some CD proteins do not play a role in cell signaling, but have other functions, such as cell adhesion. CD for humans is numbered up to 371 (). Nomenclature The CD nomenclature was proposed and established in the 1st International Workshop and Conference on Human Leukocyte Differentiation Antigens (HLDA), held in Paris in 1982. This system was intended for the classification of the many monoclonal antibodies (mAbs) generated by different laboratories around the world against epitopes on the surface mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
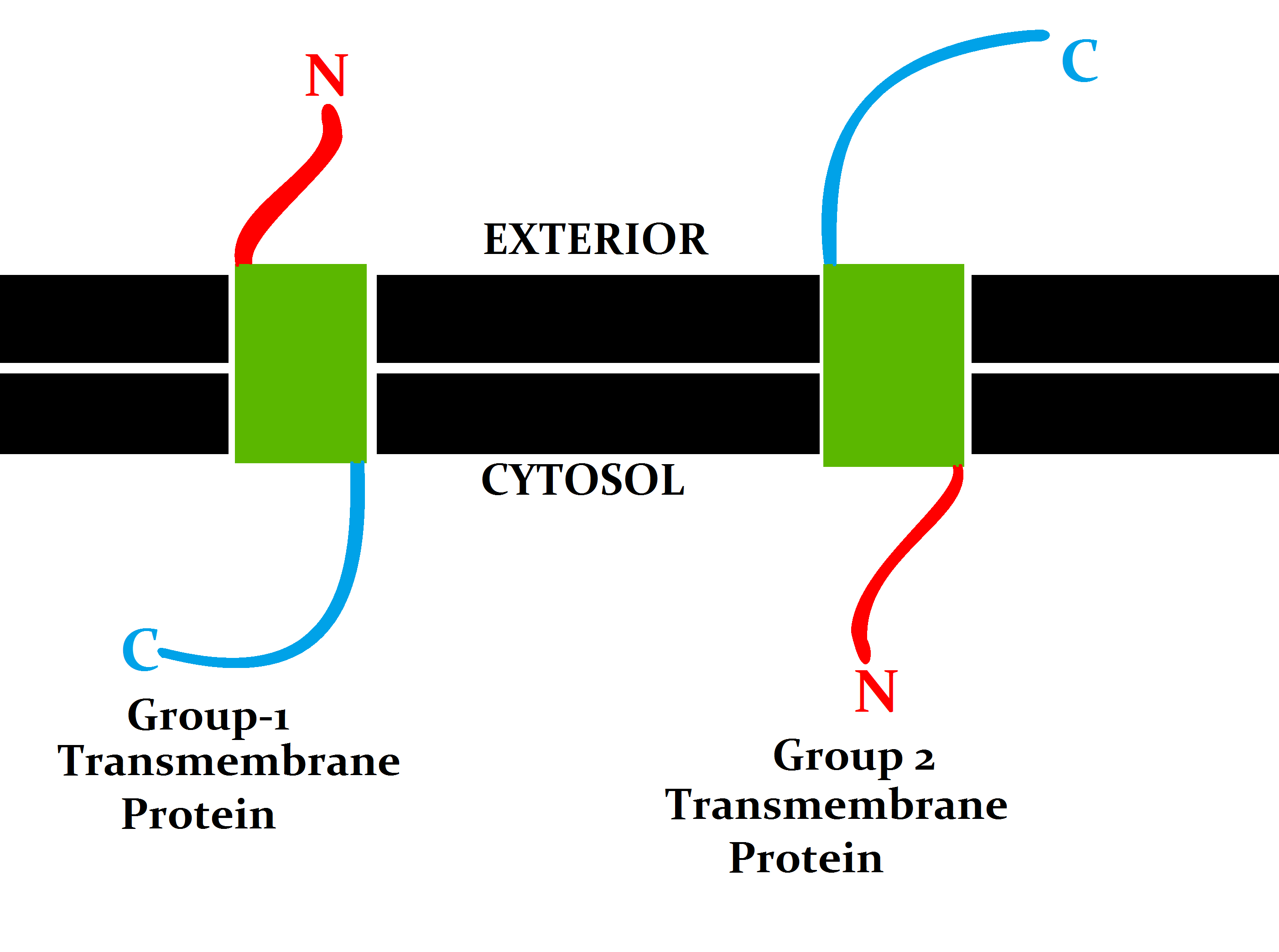
Transmembrane
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-pass membrane proteins, or as multipass membrane proteins. Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antigen
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. Antigens can be proteins, peptides (amino acid chains), polysaccharides (chains of simple sugars), lipids, or nucleic acids. Antigens exist on normal cells, cancer cells, parasites, viruses, fungus, fungi, and bacteria. Antigens are recognized by antigen receptors, including antibodies and T-cell receptors. Diverse antigen receptors are made by cells of the immune system so that each cell has a specificity for a single antigen. Upon exposure to an antigen, only the lymphocytes that recognize that antigen are activated and expanded, a process known as clonal selection. In most cases, antibodies are ''antigen-specific'', meaning that an antibody can only react to and bind one specific antigen; in some instances, however, antibodies may cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atypical Hemolytic-uremic Syndrome
Atypical hemolytic uremic syndrome (aHUS), also known as complement-mediated hemolytic uremic syndrome (not to be confused with hemolytic–uremic syndrome), is an extremely rare, life-threatening, progressive disease that frequently has a genetic component. In most cases, it can be effectively controlled by interruption of the complement cascade. Particular monoclonal antibodies, discussed later in the article, have proven efficacy in many cases. aHUS is usually caused by chronic, uncontrolled activation of the complement system, a branch of the body's immune system that destroys and removes foreign particles. The disease affects both children and adults and is characterized by systemic thrombotic microangiopathy (TMA), the formation of blood clots in small blood vessels throughout the body, which can lead to stroke, heart attack, kidney failure, and death. The complement system activation may be due to mutations in the complement regulatory proteins (factor H, factor I, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoprotein
Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase B2
Carboxypeptidase B2 (CPB2), also known as carboxypeptidase U (CPU), plasma carboxypeptidase B (pCPB) or thrombin-activatable fibrinolysis inhibitor (TAFI), is an enzyme that, in humans, is encoded by the gene ''CPB2''. Function CPB2 is synthesized by the liverKaushansky K, Lichtman M, Beutler E, Kipps T, Prchal J, Seligsohn U. (2010; edition 8: pages 1833-1834 and 2040-2041) ''Williams Hematology''. McGraw-Hill. and circulates in the plasma as a plasminogen-bound zymogen. When it is activated by proteolysis at residue Arg92 by the thrombin/ thrombomodulin complex, CPB2 exhibits carboxypeptidase activity. Activated CPB2 reduces fibrinolysis by removing the fibrin C-terminal residues that are important for the binding and activation of plasminogen. Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticoagulant
An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood. As a class of medications, anticoagulants are used in therapy for thrombotic disorders. Oral anticoagulants (OACs) are taken by many people in pill or tablet form, and various intravenous anticoagulant dosage forms are used in hospitals. Some anticoagulants are used in medical equipment, such as sample tubes, blood transfusion bags, heart–lung machines, and dialysis equipment. One of the first anticoagulants, warfarin, was initially approved as a rodenticide. Anticoagulants are closely related to antiplatelet drugs and thrombolytic drugs by manipulating the various pathways of blood coagulation. Specifically, antiplatelet drugs inhibit platelet agg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein C
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIV, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the ''PROC'' gene, which is found on chromosome 2. The zymogenic form of protein C is a vitamin K-dependent glycoprotein that circulates in blood plasma. Its structure is that of a two-chain polypeptide consisting of a light chain and a heavy chain connected by a disulfide bond. The protein C zymogen is activated when it binds to thrombin, another protein heavily involved in coagulation, and protein C' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be classified into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a " prosthetic group", which consists of a coenzyme that is tightly (or even covalently and, therefore, permanently) bound to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |