|
TDMA (drug)
TDMA is a bioisosteric analogue of 3,4-methylenedioxy-''N''-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzothiadiazole ring. ODMA and SeDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024. MDMA and 3,4-methylenedioxyamphetamine (MDA) are well-known serotonergic neurotoxins that damage serotonergic neurons in the brain. However, MDMA and MDA injected directly into the brain have been found to not produce sero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin–norepinephrine–dopamine Releasing Agent
A serotonin–norepinephrine–dopamine releasing agent (SNDRA), also known as a triple releasing agent (TRA), is a type of drug which induces the release of serotonin, norepinephrine/epinephrine, and dopamine in the brain and body. SNDRAs produce euphoriant, entactogen, and psychostimulant effects, and are almost exclusively encountered as recreational drugs. A closely related type of drug is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI). Examples of SNDRAs Examples of SNDRAs include specific amphetamines such as MDMA, MDA, 4-methylamphetamine, methamphetamine (in high doses), certain substituted benzofurans such as 5-APB and 6-APB, naphthylisopropylamine; cathinones such as mephedrone and methylone; tryptamines such as αMT and αET; along with agents of other chemical classes such as 4,4'-DMAR, and 5-IAI.Bruce E. Blough, Richard Rothman, Antonio Landavazo, Kevin M. Page, Ann Marie Decker. Phenylmorpholines and analogues thereof. US Patent 2013/0 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2C Receptor
The 5-HT2C receptor is a subtype of the 5-HT2 receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, it is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. ''HTR2C'' denotes the human gene encoding for the receptor, that in humans is located on the X chromosome. As males have one copy of the gene and females have one of the two copies of the gene repressed, polymorphisms at this receptor can affect the two sexes to differing extent. Structure At the cell surface the receptor exists as a homodimer. The crystal structure has been known since 2018. Distribution 5-HT2C receptors are located mainly in the choroid plexus, and in rats is also found in many other brain regions in high concentrations, including parts of the hippocampus, anterior olfactory nucleus, substantia nigra, several brainstem nuclei, amygdala, subthalamic nucleus and lateral habenula. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell (biology), cell. The reactants, products, and Metabolic intermediate, intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product (chemistry), product of one enzyme acts as the substrate (chemistry), substrate for the next. However, side products are considered waste and removed from the cell. Different metabolic pathways function in the position within a Eukaryotic Cell, eukaryotic cell and the significance of the pathway in the given compartment of the cell. For instance, the electron transport chain and oxidative phosphorylation all take place in the mitochondrial membrane. In contrast, glycolysis, pentose phosphate pathway, and Fatty acid synthesis, fatty acid biosynthesis all occur in the cytosol of a cell. There are two types of metabolic pathw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
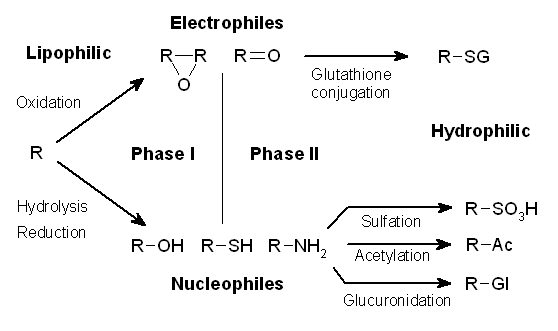
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics. Metabolism is one of the stages (see ADME) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body. The metabolism of pharmaceutical drugs is an important as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Demethylation
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms. The counterpart of demethylation is methylation. In biochemistry : Demethylation is relevant to epigenetics. Demethylation of DNA is catalyst, catalyzed by demethylases. These enzymes oxidize N-methyl groups, which occur in histones, in lysine derivatives, and in some forms of DNA. :R2N-CH3 + O → R2N-H + CH2O One family of such oxidative enzymes is the cytochrome P450. Alpha-ketoglutarate-dependent hydroxylases are also active for demethylation of DNA, operating by a similar stoichiometry. These reactions, which proceed via hydroxylation, exploit the slightly weakened Carbon–hydrogen bond, C-H bonds of methylamines and methyl ethers. Demethylation of some sterols are steps in the biosynthesis of testosterone and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and interactions with other organisms (e.g. pigments, odorants, and pheromones). A primary metabolite is directly involved in normal "growth", development, and reproduction. Ethylene exemplifies a primary metabolite produced large-scale by industrial microbiology. A secondary metabolite is not directly involved in those processes, but usually has an important ecological function. Examples include antibiotics and pigments such as resins and terpenes etc. Some antibiotics use primary metabolites as precursors, such as actinomycin, which is created from the primary metabolite tryptophan. Some sugars are metabolites, such as fructose or glucose, which ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
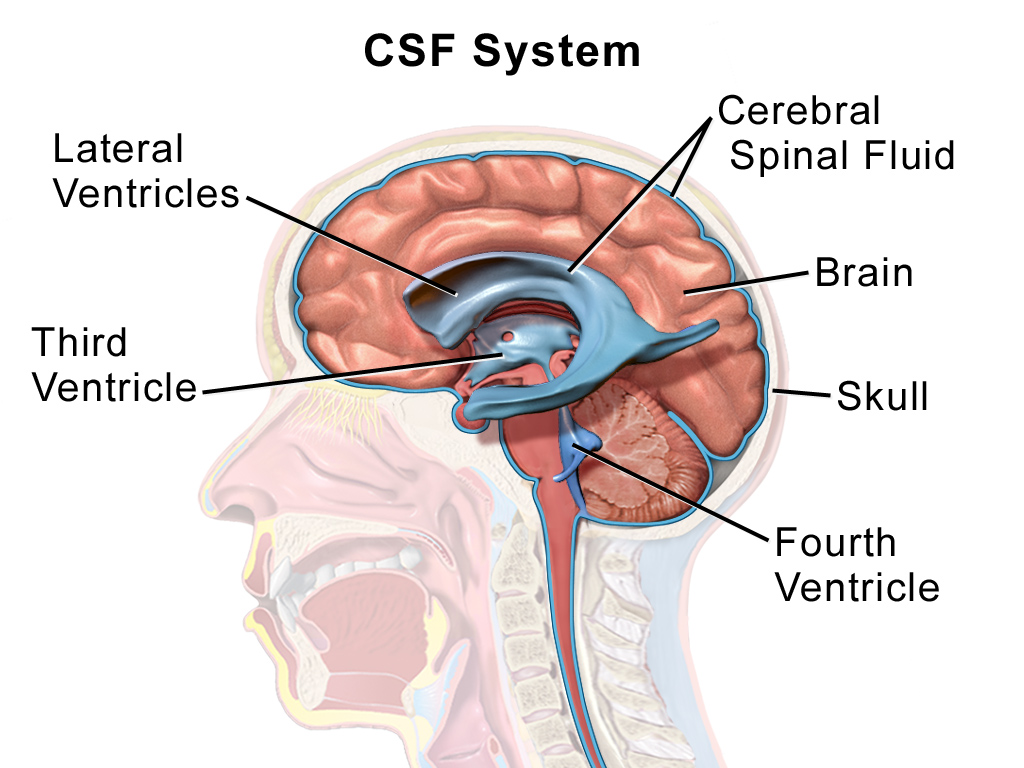
Intracerebroventricular Injection
Intracerebroventricular injection (often abbreviated as ICV injection) is a route of administration for drugs via injection into the cerebral ventricles so that it reaches the cerebrospinal fluid (CSF). This route of administration is often used to bypass the blood-brain barrier because it can prevent important medications from reaching the central nervous system. This injection method is widely used in diseased mice models to study the effect of drugs, plasmid DNA, and viral vectors on the central nervous system. In humans, ICV injection can be used for the administration of drugs for various reasons. Examples include the treatment of Spinal Muscular Atrophy (SMA), the administration of chemotherapy in gliomas, and the administration of drugs for long-term pain management. ICV injection is also used in the creation of diseased animal models specifically to model neurological disorders. Uses Creation of Animal Models Intracerebroventricular injection has been used to inject ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brain
The brain is an organ (biology), organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head (cephalization), usually near organs for special senses such as visual perception, vision, hearing, and olfaction. Being the most specialized organ, it is responsible for receiving information from the sensory nervous system, processing that information (thought, cognition, and intelligence) and the coordination of motor control (muscle activity and endocrine system). While invertebrate brains arise from paired segmental ganglia (each of which is only responsible for the respective segmentation (biology), body segment) of the ventral nerve cord, vertebrate brains develop axially from the midline dorsal nerve cord as a brain vesicle, vesicular enlargement at the rostral (anatomical term), rostral end of the neural tube, with centralized control over all body segments. All vertebr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonergic Cell Groups
A serotonergic substance, medication, or receptor protein is one that affects neurotransmission pathways that involve serotonin, as follows: * Serotonergic drugs ** Serotonin receptor agonists ** Serotonin receptor antagonists ** Serotonin reuptake inhibitors ** Serotonin releasing agents ** Serotonergic psychedelic Psychedelics are a subclass of hallucinogenic drugs whose primary effect is to trigger non-ordinary mental states (known as psychedelic experiences or "trips") and a perceived "expansion of consciousness". Also referred to as classic halluci ...s * Serotonergic cells ** Serotonergic cell groups {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurotoxicity
Neurotoxicity is a form of toxicity in which a biological, chemical, or physical agent produces an adverse effect on the structure or function of the central and/or peripheral nervous system. It occurs when exposure to a substance – specifically, a neurotoxin or neurotoxicant– alters the normal activity of the nervous system in such a way as to cause permanent or reversible damage to nervous tissue. This can eventually disrupt or even kill neurons, which are cells that transmit and process signals in the brain and other parts of the nervous system. Neurotoxicity can result from organ transplants, radiation treatment, certain drug therapies, recreational drug use, exposure to heavy metals, bites from certain species of venomous snakes, pesticides, certain industrial cleaning solvents, fuels and certain naturally occurring substances. Symptoms may appear immediately after exposure or be delayed. They may include limb weakness or numbness, loss of memory, vision, and/or inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonergic Neurotoxin
A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine. Examples of monoamine neurotoxins include the serotonergic neurotoxins ''para''-chloroamphetamine (PCA), methylenedioxymethamphetamine (MDMA), and 5,7-dihydroxytryptamine (5,7-DHT); the dopaminergic neurotoxins oxidopamine (6-hydroxydopamine), MPTP, and methamphetamine; and the noradrenergic neurotoxins oxidopamine and DSP-4. In the case of serotonergic neurotoxins like MDMA, research suggests that simultaneous induction of serotonin and dopamine release, serotonin depletion, dopamine uptake and metabolism, hyperthermia, oxidative stress and antioxidant depletion, and/or drug metabolites may all be involved in the neurotoxicity. On the other hand, there is evidence that drug metabolites may not be involved. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |