|
TAAR9
Trace amine-associated receptor 9 is a protein that in humans is encoded by the ''TAAR9'' gene. TAAR9 is a member of a large family of rhodopsin G protein–coupled receptors (GPCRs, or GPRs). GPCRs contain 7 transmembrane domains and transduce extracellular signals through heterotrimeric G proteins. upplied by OMIMref name="entrez"/> N-Methyl piperidine is a ligand of TAAR9 associated with aversive behavior in mice. N,N-dimethylcyclohexylamine is an additional binding agonist that also activaes TAAR7 variants. TAAR9 gene deletion in rats leads to significantly decreased low-density lipoprotein cholesterol levels in the blood. See also * Trace amine-associated receptor Trace amine-associated receptors (TAARs), sometimes referred to as trace amine receptors (TAs or TARs), are a class of G protein-coupled receptors that were discovered in 2001. TAAR1, the first of six functional human TAARs, has gained considerab ... References Further reading * * G protein- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine-associated Receptor
Trace amine-associated receptors (TAARs), sometimes referred to as trace amine receptors (TAs or TARs), are a class of G protein-coupled receptors that were discovered in 2001. TAAR1, the first of six functional human TAARs, has gained considerable interest in academic and proprietary pharmaceutical research due to its role as the endogenous Receptor (biochemistry), receptor for the trace amines phenethylamine, tyramine, and tryptamine – metabolite, metabolic derivatives of the amino acids phenylalanine, tyrosine and tryptophan, respectively – ephedrine, as well as the synthetic psychostimulants, amphetamine, methamphetamine and methylenedioxymethamphetamine (MDMA, ecstasy). In 2004, it was shown that mammalian TAAR1 is also a receptor for thyronamines, decarboxylation, decarboxylated and iodine, deiodinated relatives of thyroid hormones. TAAR2–TAAR9 function as olfactory receptors for volatility (chemistry), volatile amine odorants in vertebrates. Animal TAAR co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and non-coding genes. During gene expression (the synthesis of Gene product, RNA or protein from a gene), DNA is first transcription (biology), copied into RNA. RNA can be non-coding RNA, directly functional or be the intermediate protein biosynthesis, template for the synthesis of a protein. The transmission of genes to an organism's offspring, is the basis of the inheritance of phenotypic traits from one generation to the next. These genes make up different DNA sequences, together called a genotype, that is specific to every given individual, within the gene pool of the population (biology), population of a given species. The genotype, along with environmental and developmental factors, ultimately determines the phenotype ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the ''RHO'' gene and a G-protein-coupled receptor (GPCR). It is a light-sensitive receptor protein that triggers visual phototransduction in rod cells. Rhodopsin mediates dim light vision and thus is extremely sensitive to light. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is fully regenerated in about 30 minutes, after which the rods are more sensitive. Defects in the rhodopsin gene cause eye diseases such as retinitis pigmentosa and congenital stationary night blindness. History Rhodopsin was discovered by Franz Christian Boll in 1876. The name rhodopsin derives from Ancient Greek () for "rose", due to its pinkish color, and () for "sight". It was coined in 1878 by the German physiologist Wilhelm Friedrich Kühne (1837–1900). When George Wald discovered that rhodopsin is a holoprotein, consisting of retinal and an apoprotein, he called it opsin, which tod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
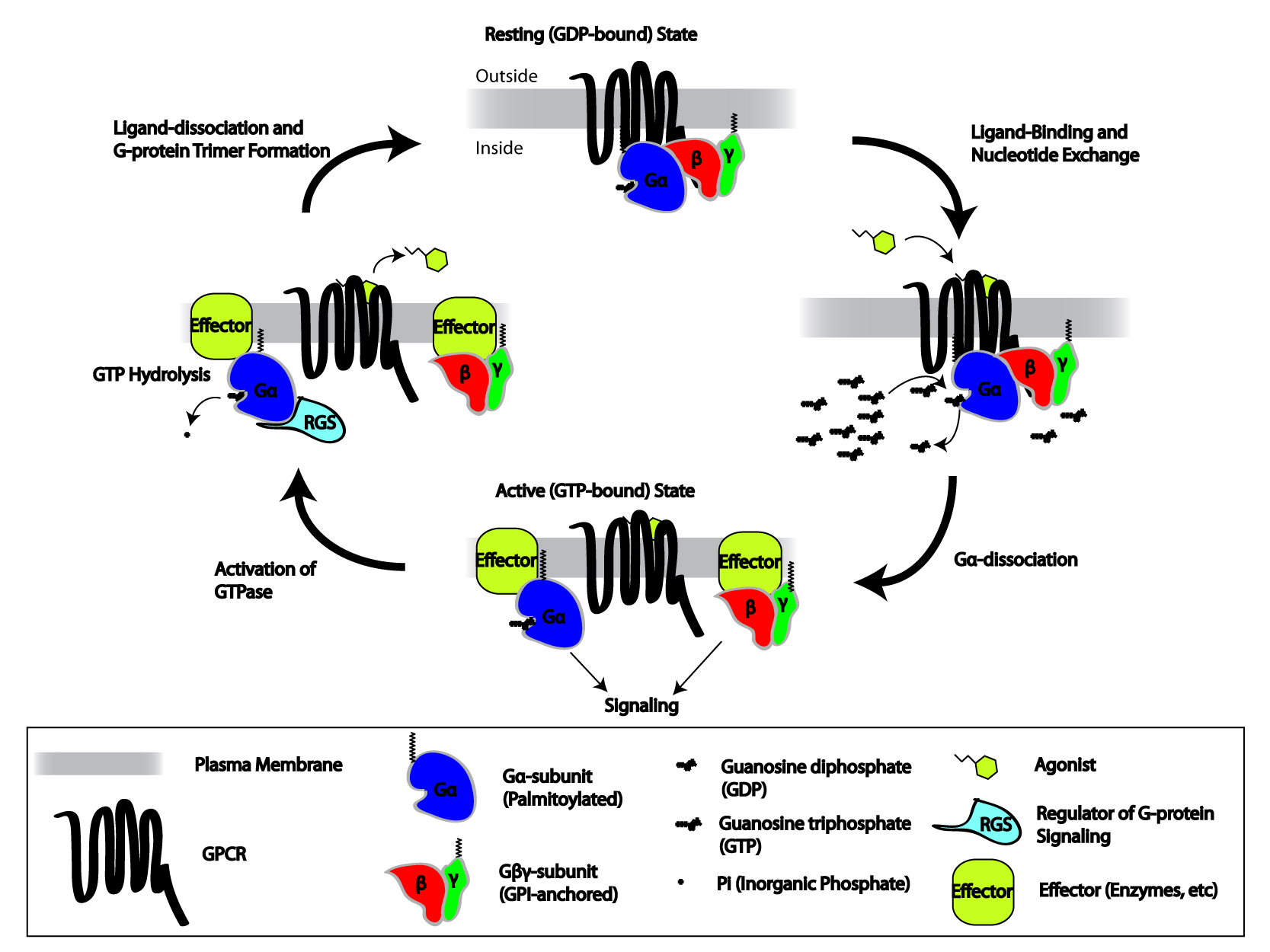
G Protein–coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops (three extracellular loops interacting with ligand molecules, three intracellular loops interacting with G proteins, an N-terminal extracellular region and a C-terminal intracellular region) of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) licence/ref> Ligands can bind either to the extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotrimeric G Protein
Heterotrimeric G protein, also sometimes referred to as the ''"large" G proteins'' (as opposed to the subclass of smaller, monomeric small GTPases) are membrane-associated G proteins that form a Heteromer, heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors (GPCR), directly. These G proteins are made up of ''alpha'' (α), ''beta'' (β) and ''gamma'' (γ) Protein subunit, subunits. The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein. When ligands bind a GPCR, the GPCR acquires GEF (guanine nucleotide exchange factor) ability, which activates the G-protein by exchanging the GDP on the ''alpha'' subunit to GTP. The binding of GTP to the ''alpha'' subunit results in a structural change and its dissociation from the rest of the G-protein. Generally, the ''alp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |