|
Supercritical Adsorption Figure5
Supercritical may refer to: Physics and technology Condensed matter physics * Critical temperature, TC, a temperature above which distinct liquid and gas phases do not exist for a given material ** Supercritical drying, a process used to remove liquid in a precisely controlled way, similar to freeze drying ** Supercritical fluid, a substance at a temperature and pressure above its thermodynamic critical point: *** Supercritical carbon dioxide: **** Supercritical fluid chromatography, a form of liquid chromatography using supercritical carbon dioxide as the mobile phase ***Supercritical water: **** Supercritical steam generator, a steam generator operating above the critical point of water, hence having no water–steam separation **** Supercritical water oxidation or SCWO, a process that occurs in water at temperatures and pressures above a mixture's thermodynamic critical point **** Supercritical water reactor (SCWR), a Generation IV nuclear reactor concept that uses supercritica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Temperature
Critical or Critically may refer to: *Critical, or critical but stable, medical states **Critical, or intensive care medicine * Critical juncture, a discontinuous change studied in the social sciences. * Critical Software, a company specializing in mission and business critical information systems *Critical theory, a school of thought that critiques society and culture by applying knowledge from the social sciences and the humanities * Critically endangered, a risk status for wild species * Criticality (status), the condition of sustaining a nuclear chain reaction Art, entertainment, and media * ''Critical'' (novel), a medical thriller written by Robin Cook * ''Critical'' (TV series), a Sky 1 TV series * "Critical" (''Person of Interest''), an episode of the American television drama series ''Person of Interest'' *"Critical", a 1999 single by Zion I People *Cr1TiKaL (born 1994), an American YouTuber and Twitch streamer See also *Critic * Criticality (other) *Critical Co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Drying
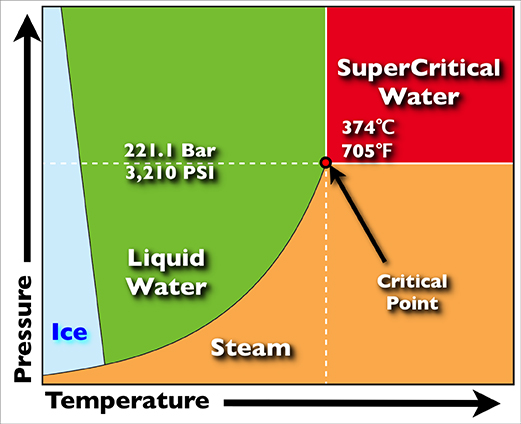
Supercritical drying, also known as critical point drying, is a process to remove liquid in a precise and controlled way. It is useful in the production of microelectromechanical systems (MEMS), the drying of spices, the production of aerogel, the decaffeination of coffee and in the preparation of biological specimens. Phase diagram As the substance in a liquid body crosses the boundary from liquid to gas (see green arrow in phase diagram), the liquid changes into gas at a finite rate, while the amount of liquid decreases. When this happens within a heterogeneous environment, surface tension in the liquid body pulls against any solid structures the liquid might be in contact with. Delicate structures such as cell walls, the dendrites in silica gel, and the tiny machinery of microelectromechanical devices, tend to be broken apart by this surface tension as the liquid–gas–solid junction moves by. To avoid this, the sample can be brought via two possible alternate paths f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Fluid
A supercritical fluid (SCF) is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist, but below the pressure required to compress it into a solid. It can effuse through porous solids like a gas, overcoming the mass transfer limitations that slow liquid transport through such materials. SCF are much superior to gases in their ability to dissolve materials like liquids or solids. Also, near the critical point, small changes in pressure or temperature result in large changes in density, allowing many properties of a supercritical fluid to be "fine-tuned". Supercritical fluids occur in the atmospheres of the gas giants Jupiter and Saturn, the terrestrial planet Venus, and probably in those of the ice giants Uranus and Neptune. Supercritical water is found on Earth, such as the water issuing from black smokers, a type of underwater hydrothermal vent. They are used as a substitute for organic solvents in a range o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Carbon Dioxide
Supercritical carbon dioxide (s) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure. Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as a solid called dry ice when cooled and/or pressurised sufficiently. If the temperature and pressure are both increased from STP to be at or above the critical point for carbon dioxide, it can adopt properties midway between a gas and a liquid. More specifically, it behaves as a supercritical fluid above its critical temperature () and critical pressure (), expanding to fill its container like a gas but with a density like that of a liquid. Supercritical is becoming an important commercial and industrial solvent due to its role in chemical extraction in addition to its relatively low toxicity and environmental impact. The relatively low temperature of the process and the stability of also allows most compounds to be extracted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercritical Fluid Chromatography
Supercritical fluid chromatography (SFC) is a form of normal phase chromatography that uses a supercritical fluid such as carbon dioxide as the mobile phase. It is used for the analysis and purification of low to moderate molecular weight, thermally labile molecules and can also be used for the separation of chiral compounds. Principles are similar to those of high performance liquid chromatography (HPLC), however SFC typically utilizes carbon dioxide as the mobile phase; therefore the entire chromatographic flow path must be pressurized. Because the supercritical phase represents a state in which liquid and gas properties converge, supercritical fluid chromatography is sometimes called convergence chromatography. Applications SFC is used in industry primarily for separation of chiral molecules, and uses the same columns as standard HPLC systems. SFC is now commonly used for achiral separations and purifications in the pharmaceutical industry. Apparatus SFC with CO2 utilizes carbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |