|
Sulfur Fluoride s, some of which are valence isoelectronic with sulfur fluorides
{{Chemistry index ...
Sulfur fluoride may refer to any of the following sulfur fluorides: *Sulfur hexafluoride, SF6 *Disulfur decafluoride, S2F10 *Sulfur tetrafluoride, SF4 * Disulfur tetrafluoride, S2F4 *Sulfur difluoride, SF2 * Disulfur difluoride, S2F2 * Thiothionyl fluoride, S2F2 (second isomer) * 1,3-Difluoro-trisulfane-1,1-difluoride, S3F4 See also *Chlorine oxide Chlorine and oxygen can bond in a number of ways: * chlorine monoxide radical, , chlorine (II) oxide radical * chloroperoxyl radical, , chlorine (II) peroxide radical *chlorine dioxide, , chlorine (IV) oxide * chlorine trioxide radical, , chlorin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Hexafluoride
Sulfur hexafluoride or sulphur hexafluoride ( British spelling) is an inorganic compound with the formula SF6. It is a colorless, odorless, non-flammable, and non-toxic gas. has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule. Typical for a nonpolar gas, is poorly soluble in water but quite soluble in nonpolar organic solvents. It has a density of 6.12 g/L at sea level conditions, considerably higher than the density of air (1.225 g/L). It is generally stored and transported as a liquefied compressed gas. has 23,500 times greater global warming potential (GWP) than as a greenhouse gas (over a 100-year time-frame) but exists in relatively minor concentrations in the atmosphere. Its concentration in Earth's troposphere reached 12.06 parts per trillion (ppt) in February 2025, rising at 0.4 ppt/year. The increase since 1980 is driven in large part by the expanding electric power sector, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Decafluoride
Disulfur decafluoride is a chemical compound with the formula . It was discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. The two sulfur atoms are connected by a single bond. In the molecule, the oxidation state of each sulfur atoms is +5, but their valency is 6 (they are hexavalent). is highly toxic, with toxicity four times that of phosgene. It is a colorless liquid with a burnt match smell similar to sulfur dioxide. Production Disulfur decafluoride is produced by photolysis of : : Disulfur decafluoride arises by the decomposition of sulfur hexafluoride. It is produced by the electrical decomposition of sulfur hexafluoride ()—an essentially inert insulator used in high voltage systems such as transmission lines, substations and switchgear. is also made during the production of . Properties The S-S bond dissociation energy is 305 ± 21 kJ/mol, about 80 kJ/mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Tetrafluoride
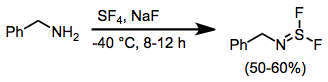
Sulfur tetrafluoride is a chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the +4 oxidation state, with one lone pair of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Tetrafluoride
1,1,1,2-tetrafluorodisulfane, also known as 1,2-difluorodisulfane 1,1-difluoride or just difluorodisulfanedifluoride (FSSF3) is an unstable molecular compound of fluorine and sulfur. The molecule has a pair of sulfur atoms, with one fluorine atom on one sulfur, and three fluorine atoms on the other. It has the uncommon property that all the bond lengths are different. The bond strength is not correlated with bond length but is inversely correlated with the force constant ( Badger's rule). The molecule can be considered as sulfur tetrafluoride in which a sulfur atom is inserted into a S-F bond. Atoms are labelled with the sulfur atom connected to three fluorine atoms as S''hyp'' (for hypervalent) and S''top''. The fluorine atoms are labelled F''top'' attached to S''top'', and on the hypervalent S atom: F''cis'', the closest F atom to F''top'', F''trans'' the furthest away F atom from F''top'', and F''eq'' Carlowitz first determined the structure in 1983. F''eq'' is 90° from F''t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Difluoride
Sulfur difluoride is an inorganic compound with the chemical formula SF2. It can be generated by the reaction of sulfur dichloride and potassium fluoride or mercury(II) fluoride at low pressures: :SCl2 + 2 KF → SF2 + 2 KCl :SCl2 + HgF2 → SF2 + HgCl2 The F−S−F bond angle is 98°, and the length of S−F bond is 159 pm. The compound is highly unstable, dimerising to FSSF3. This unsymmetrical isomer of S2F4 is proposed to arise via insertion of SF2 into the S−F bond of a second molecule SF2: It can also be formed from oxygen difluoride and hydrogen sulfide Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist ...: :OF2 + H2S → SF2 + H2O References {{Fluorides Sulfur(II) compounds Fluorides Sulfur fluorides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfur Difluoride
Disulfur difluoride is an inorganic compound with the chemical formula . It is a halide of sulfur. Structure Disulfur difluoride has a chain structure . The angle between the and planes is 87.9°, while the angles of and are equivalent, and are equal to 108.3°. Both bonds are equivalent and their length is 163.5 pm, while the length of the bond is 189 pm. This structure is referred to as gauche, and is similar to . There is a branched isomer of disulfur difluoride, thiothionyl fluoride, with the structure . Synthesis Silver(II) fluoride can fluorinate sulfur in a strictly dry container at , and the reaction produces : : Reactions Disulfur difluoride undergoes intramolecular rearrangement in the presence of fluorides of alkali metals, yielding the isomer S=SF2: : * Decomposing to sulfur tetrafluoride and sulfur when heated to 180 °C: : * Hydrolysis: : * Reacting with sulfuric acid at 80 °C: : * Reacting with sodium hydroxide: : * Reacting with oxygen at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiothionyl Fluoride
Thiothionyl fluoride is a chemical compound of fluorine and sulfur, with the chemical formula . It is an isomer of disulfur difluoride (difluorodisulfane) . Preparation Thiothionyl fluoride can be obtained from the reaction between disulfur dichloride with potassium fluoride at about 150 °C or with mercury(II) fluoride at 20 °C. : Another possible preparation is by the reaction of nitrogen trifluoride with sulfur. : It also forms from disulfur difluoride when in contact with alkali metal fluorides. can also be synthesized with the reaction of potassium fluorosulfite and disulfur dichloride: : Properties Thiothionyl fluoride is a colorless gas. At high temperatures and pressures, it decomposes into sulfur tetrafluoride and sulfur. : With hydrogen fluoride, it forms sulfur tetrafluoride and hydrogen sulfide. : It condenses with sulfur difluoride Sulfur difluoride is an inorganic compound with the chemical formula SF2. It can be generated by the reaction of sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,3-Difluoro-trisulfane-1,1-difluoride
1,3-Difluoro-trisulfane-1,1-difluoride is an inorganic compound with the chemical formula . It has the structure . It is a molecular compound, consisting of sulfur in valences of 2 and 4 and fluorine. The compound consists of a chain of three sulfur atoms, with three fluorine atoms bonded to the sulfur on one end and the fourth fluorine bonded to the sulfur on the other end. It has a melting point of −62 °C and a boiling point of 94 °C. As a gas, it is unstable and breaks up to form and . is produced by the condensation of sulfur difluoride Sulfur difluoride is an inorganic compound with the chemical formula SF2. It can be generated by the reaction of sulfur dichloride and potassium fluoride or mercury(II) fluoride at low pressures: :SCl2 + 2 KF → SF2 + 2 KCl :SCl2 + HgF2 → SF2 + ... and an isomer of . The reaction : uses 6 kJ/mol. Possible isomers of its molecular formula include , which has a spontaneous fluorine migration to yield , which in turn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |