|
Sulfolobus Spindle-shaped Virus 2
''Sulfolobus'' is a genus of microorganism in the family Sulfolobaceae. It belongs to the kingdom Thermoproteati of the Archaea domain. ''Sulfolobus'' species grow in volcanic springs with optimal growth occurring at pH 2–3 and temperatures of 75–80 °C, making them acidophiles and thermophiles respectively. ''Sulfolobus'' cells are irregularly shaped and flagellar. Species of ''Sulfolobus'' are generally named after the location from which they were first isolated, e.g. ''Sulfolobus solfataricus'' (now recombined as ''Saccharolobus solfataricus)'' was first isolated in the Solfatara volcano. Other species can be found throughout the world in areas of volcanic or geothermal activity, such as geological formations called mud pots, which are also known as ''solfatare'' (plural of solfatara). ''Sulfolobus'' as a model to study the molecular mechanisms of DNA replication When the first Archaeal genome, ''Methanococcus jannaschii'', had been sequenced completely in 1996, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
STSV-1
Sulfolobus tengchongensis spindle-shaped virus 1 (STSV1) is a DNA virus of the family '' Bicaudaviridae''. It infects the hyperthermophilic archaeon '' Sulfolobus tengchongensis'' which can be found in the volcanic area of Tengchong, Baoshan City, in western Yunnan province, People's Republic of China. In 2014, Sulfolobus tengchongensis spindle-shaped virus 2 (STSV2), a relative of STSV1, also infecting ''S. tengchongensis'', has been reported. Besides ''S. tengchongensis'', STSV2 infects ''Sulfolobus islandicus'' REY15A. It has been demonstrated that STSV2 induces unprecedented gigantism of ''S. islandicus'' cells by blocking the expression of the cell division genes and arresting the cell cycle in the S phase. The diameter of infected cells increases up to 20 times, resulting in 8,000-fold increase in volume compared to noninfected cells. References External links * UCSC Sulfolobus virus STSV1 Genome Browser GatewaySTSV-1, also STSV-2, and Sulfolobus spindle-shaped virus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solfatara (volcano)
Solfatara () is a shallow volcanic crater at Pozzuoli, near Naples, part of the Phlegraean Fields () volcanic area. It is a dormant volcano, which still emits jets of steam with sulfurous fumes. The name comes from the Latin, ''Sulpha terra'', "land of sulfur", or "sulfur earth". It was formed around 4000 years ago and last erupted in 1198 with what was probably a ''phreatic'' eruption – an explosive steam-driven eruption caused when groundwater interacts with magma Magma () is the molten or semi-molten natural material from which all igneous rocks are formed. Magma (sometimes colloquially but incorrectly referred to as ''lava'') is found beneath the surface of the Earth, and evidence of magmatism has also .... The crater floor was a popular tourist attraction until 2017, as it has many fumaroles and mud pools. The area is well known for its bradyseism. The vapours had been used for medical purposes since Roman times. This volcano is where the thermoacidophil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellular Respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to transfer chemical energy from nutrients to ATP, with the flow of electrons to an electron acceptor, and then release waste products. If the electron acceptor is oxygen, the process is more specifically known as aerobic cellular respiration. If the electron acceptor is a molecule other than oxygen, this is anaerobic cellular respiration. Fermentation, which is also an anaerobic process, is not respiration, as no external electron acceptor is involved. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, producing large amounts of energy (ATP). Respiration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
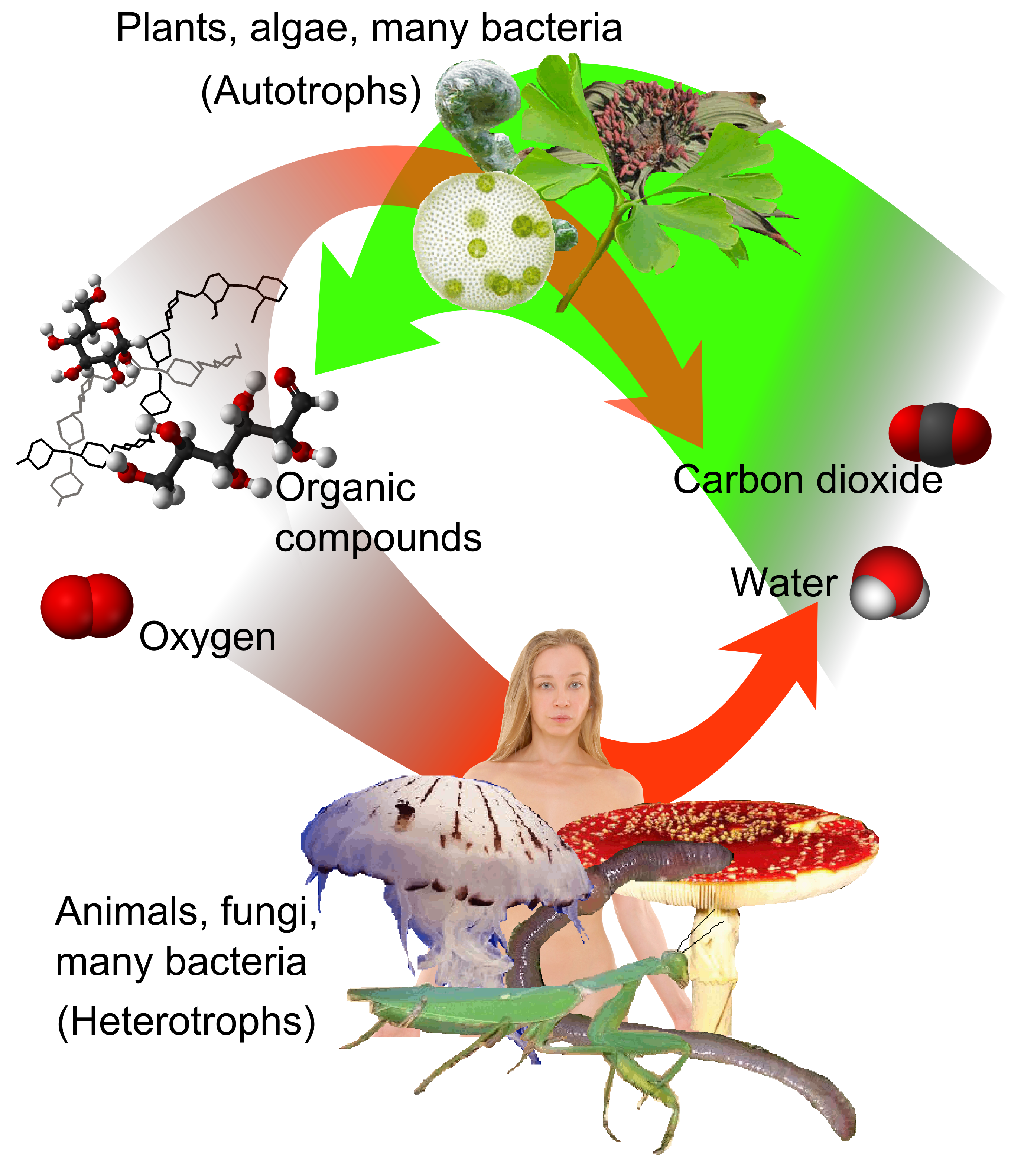
Autotroph
An autotroph is an organism that can convert Abiotic component, abiotic sources of energy into energy stored in organic compounds, which can be used by Heterotroph, other organisms. Autotrophs produce complex organic compounds (such as carbohydrates, fats, and proteins) using carbon from simple substances such as carbon dioxide,Morris, J. et al. (2019). "Biology: How Life Works", 3rd edition, W. H. Freeman. generally Photosynthesis, using energy from light or Chemosynthesis, inorganic chemical reactions. Autotrophs do not need a living source of carbon or energy and are the primary production, producers in a food chain, such as plants on land or algae in water. Autotrophs can Redox, reduce carbon dioxide to make organic compounds for biosynthesis and as stored chemical fuel. Most autotrophs use water as the reducing agent, but some can use other hydrogen compounds such as hydrogen sulfide. The primary production, primary producers can convert the energy in the light (phototrop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotroph
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but not producers. Living organisms that are heterotrophic include all animals and fungi, some bacteria and protists, and many parasitic plants. The term heterotroph arose in microbiology in 1946 as part of a classification of microorganisms based on their type of Primary nutritional groups, nutrition. The term is now used in many fields, such as ecology, in describing the food chain. Heterotrophs occupy the second and third trophic levels of the food chain while autotrophs occupy the first trophic level. Heterotrophs may be subdivided according to their energy source. If the heterotroph uses chemical energy, it is a chemotroph, chemoheterotroph (e.g., humans and mushrooms). If it uses light for energy, then it is a photoheterotroph (e.g., gre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affitin
Affitins (commercial name Nanofitins) are artificial proteins with the ability to selectively bind antigens. They are structurally derived from the DNA binding protein Sac7d, found in ''Sulfolobus acidocaldarius'', a microorganism belonging to the archaeal domain. By randomizing the amino acids on the binding surface of Sac7d and subjecting the resulting protein library to rounds of ribosome display, the affinity can be directed towards various targets, such as peptides, proteins, viruses, and bacteria. Affitins are antibody mimetics and are being developed as an alternative to antibodies as tools in biotechnology. They have also been used as specific inhibitors for various enzymes. Affitins can be utilized in biochemical purification techniques, specifically in affinity chromatography. The ability of Affitins to selectively bind antigens is used to target specific proteins. Scientists have been able to purify human immunoglobulin G (hIgG), bacterial PulD protein, and chicken eg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermostable
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature. Thermostable materials may be used industrially as fire retardants. A ''thermostable plastic'', an uncommon and unconventional term, is likely to refer to a thermosetting plastic that cannot be reshaped when heated, than to a thermoplastic that can be remelted and recast. Thermostability is also a property of some proteins. To be a thermostable protein means to be resistant to changes in protein structure due to applied heat. Thermostable proteins Most life-forms on Earth live at temperatures of less than 50 °C, commonly from 15 to 50 °C. Within these organisms are macromolecules (proteins and nucleic acids) which form the three-dimensional structures essential to their enzymatic activity. Above the native temperature of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |