|
Sulfated
Sulfation (sometimes spelled sulphation in British English) is the chemical reaction that entails the addition of SO3 group. In principle, many sulfations would involve reactions of sulfur trioxide (SO3). In practice, most sulfations are effected less directly. Regardless of the mechanism, the installation of a sulfate-like group on a substrate leads to substantial changes. Sulfation in industry Sulfation of calcium oxides Sulfation is a process used to remove "sulfur" from the combustion of fossil fuels. The goal is to minimize the pollution by the combusted gases. Combustion of sulfur-containing fuels releases sulfur dioxide, which, in the atmosphere, oxidizes to the equivalent of sulfuric acid, which is corrosive. To minimize the problem, the combustion is often conducted in the presence of calcium oxide or calcium carbonate, which, directly or indirectly, bind sulfur dioxide and some oxygen to give calcium sulfite. The net reaction is: :CaO + SO2 → CaSO3 :2 CaSO3 + O2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heparan Sulfate
Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs in a proteoglycan (HSPG, i.e. Heparan Sulfate ProteoGlycan) in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt signaling pathway, Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB (Granzyme B), and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 infection, particularly when the virus attaches with ACE2. Proteoglycans The major cell membrane HSPGs are the transmembrane syndecans and the glycosylphosphatidylinositol (GPI) anchored glypicans. Other minor forms of membrane HSPG include betaglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
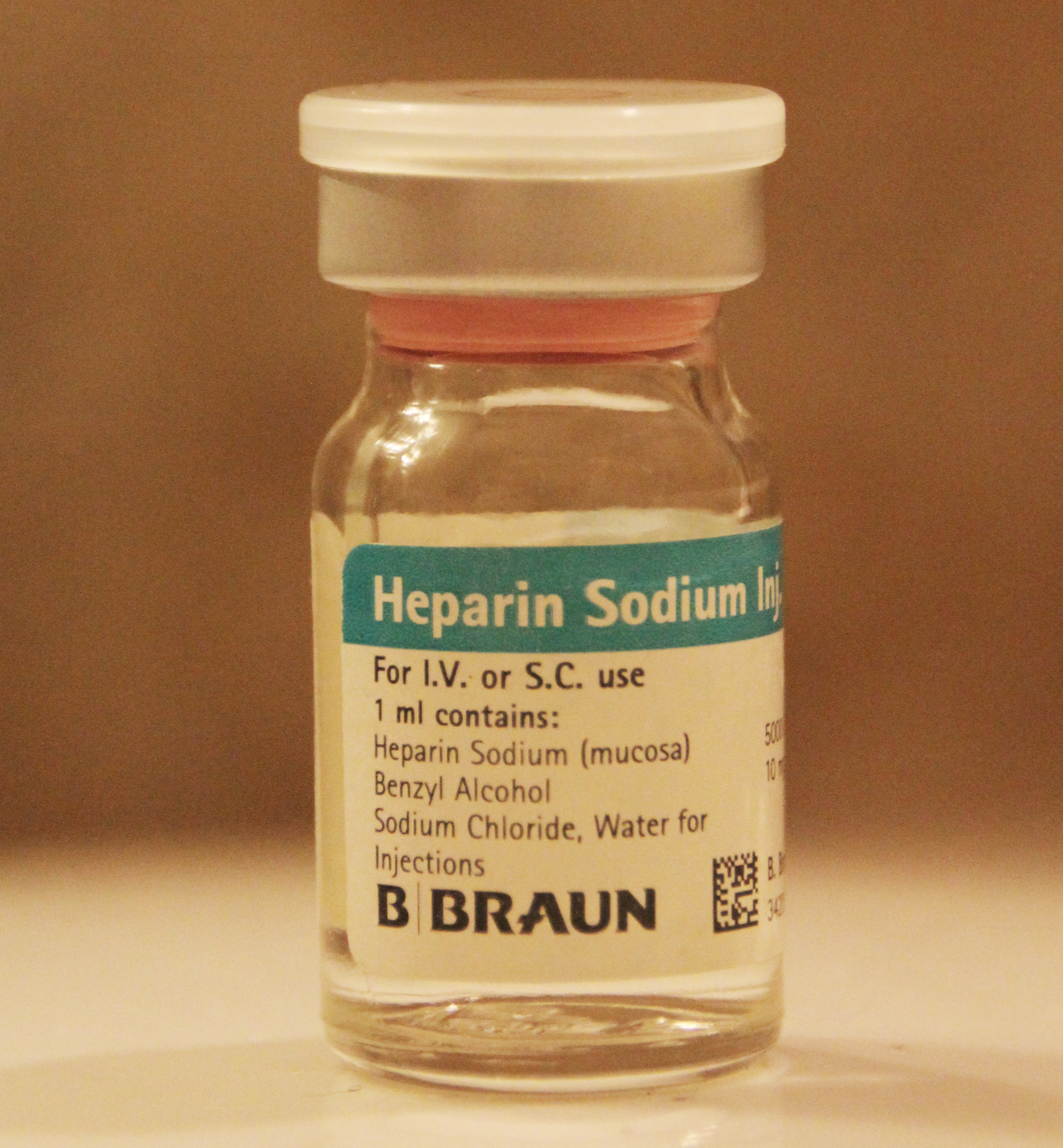
Heparin General Structure V
Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Heparin is a blood anticoagulant that increases the activity of antithrombin. It is used in the treatment of heart attacks and unstable angina. It can be given intravenously or by injection under the skin. Its anticoagulant properties make it useful to prevent blood clotting in blood specimen test tubes and kidney dialysis machines. Common side effects include bleeding, pain at the injection site, and low blood platelets. Serious side effects include heparin-induced thrombocytopenia. Greater care is needed in those with poor kidney function. Heparin is contraindicated for suspected cases of vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT) secondary to SARS-CoV-2 vaccination, as heparin may further increase the risk of bleeding in an anti-PF4/heparin complex autoimmune manner, in favor of alternative anticoagulant medications (such as argatroban or d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosaminoglycan
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units (i.e. two-sugar units). The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers. Mucopolysaccharidoses are a group of metabolic disorders in which abnormal accumulations of glycosaminoglycans occur due to enzyme deficiencies. Production Glycosaminoglycans vary greatly in molecular mass, disaccharide structure, and sulfation. This is because GAG synthesis is not template driven, as are proteins or nucleic acids, but constantly altered by processing enzymes. GAGs are classified into four groups, based on their core disaccharide structures: # H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chondroitin Sulfate
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars (N-Acetylgalactosamine, N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage, and provides much of its resistance to compressive stress, compression. Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis, although large clinical trials failed to demonstrate any symptomatic benefit of chondroitin. Fragments of chondroitin chains suspended in water elicit a fear response in many fishes, similar to schreckstoff, hypoxanthine-3N-oxide. Medical use Chondroitin is used in dietary supplements as an alternative medicine to treat osteoarthritis. It is also approved and regula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfatide
Sulfatide, also known as 3-O-sulfogalactosylceramide, SM4, or sulfated galactocerebroside, is a class of sulfolipids, specifically a class of sulfoglycolipids, which are glycolipids that contain a sulfate group. Sulfatide is synthesized primarily starting in the endoplasmic reticulum and ending in the Golgi apparatus where ceramide is converted to galactocerebroside and later sulfated to make sulfatide. Of all of the galactolipids that are found in the myelin sheath, one fifth of them are sulfatide. Sulfatide is primarily found on the extracellular leaflet of the myelin plasma membrane produced by the oligodendrocytes in the central nervous system and in the Schwann cells in the peripheral nervous system. However, sulfatide is also present on the extracellular leaflet of the plasma membrane of many cells in eukaryote, eukaryotic organisms. Since sulfatide is a multifunctional molecule, it can be used in multiple biological areas. Aside from being a membrane component, sulfatide func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead–acid Battery
The lead–acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté. It was the first type of rechargeable battery to be invented. Compared to modern rechargeable batteries, lead–acid batteries have relatively low energy density. Despite this, they are able to supply high surge currents. These features, along with their low cost, make them attractive for use in motor vehicles in order to provide the high current required by starter motors. Lead–acid batteries suffer from relatively short cycle lifespan (usually less than 500 deep cycles) and overall lifespan (due to the ''double sulfation'' in the discharged state), as well as long charging times. As they are not as expensive when compared to newer technologies, lead–acid batteries are widely used even when surge current is not important and other designs could provide higher energy densities. In 1999, lead–acid battery sales accounted for 40–50% of the value from batteries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Lauryl Sulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula and structure . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar " headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations. Physicochemical properties The critical micelle concentration (CMC) in water at 25 °C is 8.2 mM, and the aggregation number at this concentration is usually considered to be about 62. The micelle ionization fraction (α) is around 0.3 (or 30%). Applications Cleaning and hy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactolipid
Galactolipids are a type of glycolipid whose sugar group is galactose. They differ from glycosphingolipids in that they do not have nitrogen in their composition. They are the main part of plant membrane lipids where they substitute phospholipids to conserve phosphate for other essential processes. These chloroplast membranes contain a high quantity of monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG). They probably also assume a direct role in photosynthesis, as they have been found in the X-ray crystallography, X-ray structures of photosynthetic complexes. Galactolipids are more bioavailable than free fatty acids, and have been shown to exhibit COX mediated anti-inflammatory activity. Bio-guided fractionation of spinach leaves (''Spinacia oleracea'') revealed alpha-linolenic acid galactolipids (18:3, n-3) were responsible for inhibitory effects on tumor promoter-induced Epstein-Barr virus (EBV) activation. Recently, it has been demonstrated that this same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrosulfate
In chemistry, disulfate or pyrosulfate is the anion with the molecular formula . Disulfate is the IUPAC name. It has a dichromate-like structure and can be visualised as two corner-sharing SO4 tetrahedra, with a bridging oxygen atom. In this anion, sulfur has an oxidation state of +6. Disulfate is the conjugate base of the hydrogen disulfate (hydrogen pyrosulfate) ion , which in turn is the conjugate base of disulfuric acid (pyrosulfuric acid). Role in sulfation Industrial production of sulfate ester-based surfactants involves the reaction (sulfation) of fatty alcohols with sulfur trioxide. For example, dodecyl alcohol is sulfated using sulfur trioxide. The reaction proceeds by initial formation of the pyrosulfate: : : Several million tons are produced annually. See also * Potassium pyrosulfate * Sodium pyrosulfate * Pyrophosphate * Pyrocarbonate A dicarbonate, also known as a pyrocarbonate, is a chemical containing the divalent or functional group, which consists o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Dodecylsulfate
Sodium dodecyl sulfate (SDS) or sodium lauryl sulfate (SLS), sometimes written sodium laurilsulfate, is an organic compound with the formula and structure . It is an anionic surfactant used in many cleaning and hygiene products. This compound is the sodium salt of the 12-carbon organosulfate. Its hydrocarbon tail combined with a polar " headgroup" give the compound amphiphilic properties that make it useful as a detergent. SDS is also component of mixtures produced from inexpensive coconut and palm oils. SDS is a common component of many domestic cleaning, personal hygiene and cosmetic, pharmaceutical, and food products, as well as of industrial and commercial cleaning and product formulations. Physicochemical properties The critical micelle concentration (CMC) in water at 25 °C is 8.2 mM, and the aggregation number at this concentration is usually considered to be about 62. The micelle ionization fraction (α) is around 0.3 (or 30%). Applications Cleaning and hygien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfolipid
Sulfolipids are a class of lipids which possess a sulfur-containing functional group. An abundant sulfolipid is sulfoquinovosyl diacylglycerol, which is composed of a glycoside of sulfoquinovose and diacylglycerol. In plants, sulfoquinovosyl diacylglycerides (SQDG) are important members of the sulfur cycle. Other important sulfolipids include sulfatide and seminolipid, each of which are sulfated glycolipids. Sulfolipids have been implicated in the functions of two of the core components of the photosynthetic electron transport chain and while not necessarily essential, might have a protective function when the photosynthetic apparatus is under stress. See also * Sulfatide * Galactolipid *Phospholipid Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ... * Glycolipid Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |