|
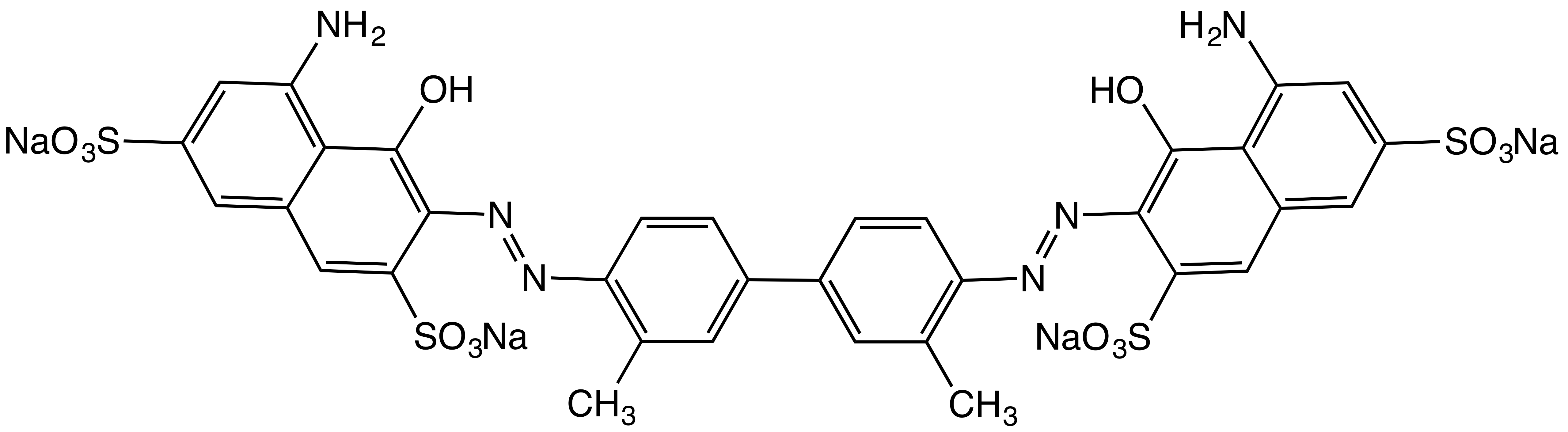
Sulfarsazene
Sulfarsazene is a chemical compound with the formula , a metal indicator. Uses Sulfarsazene is used as a metal indicator for the spectrophotometric and titrimetric determination of Pb2+ ions at pH 9.8–10.0 and Zn2+ at pH 9.3–9.6 (color transition from orange-pink to lemon yellow).{{cite journal , last1=Toroptsev , first1=Academician I. V. , last2=Eshchenko , first2=V. A. , title=Histochemical detection of zinc by means of sulfarsazene , journal=Bulletin of Experimental Biology and Medicine , date=1 September 1971 , volume=72 , issue=3 , pages=1097–1099 , doi=10.1007/BF00802415 , url=https://link.springer.com/article/10.1007/BF00802415 , access-date=18 April 2025 , language=en , issn=1573-8221, url-access=subscription Physical properties Sulfarsazene is soluble in water, easily soluble in an aqueous solution of sodium tetraborate, slightly soluble in 95% alcohol, practically insoluble in acetone, chloroform, benzene. See also * Bromothymol blue *Litmus *Methyl orange *Pheno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PH Indicator
A pH indicator is a halochromism, halochromic chemical compound added in small amounts to a Solution (chemistry), solution so the pH (acidity or Base (chemistry), basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, a pH indicator is a Chemical substance, chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Acid-base reaction theories, Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C (Standard conditions for temperature and pressure#Standard laboratory conditions, standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring Organic compound, organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PH Indicators
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C ( standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring organic compounds are weak electrolytes, such as carboxylic acids and amines, pH indicators find many applications in biology and analytical chemistry. Moreover, pH indicators form one of the three main ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Orange
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the p''K''a of a mid strength acid, it is usually used in titration of strong acids in weak bases that reach the equivalence point at a pH of 3.1-4.4. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity. Indicator colors In a solution that decreases in acidity, methyl orange moves from the color red to orange and finally to yellow with the opposite occurring for a solution increasing in acidity. This color change from yellow to red occurs because the protons in the acidic solution react with the N=N bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Routledge
Routledge ( ) is a British multinational corporation, multinational publisher. It was founded in 1836 by George Routledge, and specialises in providing academic books, academic journals, journals and online resources in the fields of the humanities, behavioral science, behavioural science, education, law, and social science. The company publishes approximately 1,800 journals and 5,000 new books each year and their backlist encompasses over 140,000 titles. Routledge is claimed to be the largest global academic publisher within humanities and social sciences. In 1998, Routledge became a subdivision and Imprint (trade name), imprint of its former rival, Taylor & Francis, Taylor & Francis Group (T&F), as a result of a £90-million acquisition deal from Cinven, a venture capital group which had purchased it two years previously for £25 million. Following the merger of Informa and T&F in 2004, Routledge became a publishing unit and major imprint within the Informa "academic publishing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Pigments
Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product of decay, or is composed of organic compounds * Organic compound, a compound that contains carbon ** Organic chemistry, chemistry involving organic compounds Farming, certification and products * Organic farming, agriculture conducted according to certain standards, especially the use of stated methods of fertilization and pest control * Organic certification, accreditation process for producers of organically-farmed products * Organic horticulture, the science and art of growing fruits, vegetables, flowers, or ornamental plants by following the essential principles of organic agriculture * Organic products, "organics": ** Organic food, food produced from organic farming methods and often certified organic according to organic farming stan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food industry, food and textile industry, textile industries. Azo dyes are widely used to treat textile, textiles, leather, leather articles, and some foods. Chemically related derivatives of azo dyes include #Azo pigments, azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Compounds
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule. Aromatic compounds have the following general properties: * Typically unreactive * Often non polar and hydrophobic * High carbon-hydrogen ratio * Burn with a strong sooty yellow flame, due to high C:H ratio * Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoarsenic Compounds
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known. History 140px, Cacodyl (tetramethyldiarsine) was one of the first organoarsenic compounds. Surprising for an area now considered of minor importance, organoarsenic chemistry played a prominent role in chemistry's history. The oldest known organoarsenic compound, the foul smelling cacodyl was reported in "cacodyl" (1760) and is sometimes classified as the first synthetic organometallic compound. The compound Salvarsan was one of the first pharmaceuticals, earning a Nobel prize for Paul Ehr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Universal Indicator
A universal indicator is a pH indicator made of a solution of several compounds that exhibit various smooth colour changes over a wide range pH values to indicate the acidity or alkalinity of solutions. A universal indicator can be in paper form or present in a form of a solution. History Although there are several commercially available universal pH indicators, most are a variation of a formula patented by Yamada in 1933. Composition A universal indicator is usually composed of water, 1-propanol, phenolphthalein, sodium hydroxide, methyl red, bromothymol blue, sodium bisulfite, and thymol blue. The colours that indicate the pH of a solution, after adding a universal indicator, are: The colors from yellow to red indicate an acidic solution, colours blue to violet indicate an alkaline solution and a green colour indicates that a solution is neutral. Wide-range pH test papers with distinct colours for each pH from 1 to 14 are also available. Colour matching char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolphthalein
Phenolphthalein ( ) is a chemical compound with the chemical formula, formula carbon, C20hydrogen, H14oxygen, O4 and is often written as "HIn", "HPh", "phph" or simply "Ph" in shorthand notation. Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colorless in acidic solutions and pink in base (chemistry), basic solutions. It belongs to the class of dyes known as phthalein dyes. Phenolphthalein is slightly soluble in water and usually is dissolved in Alcohol (chemistry), alcohols in experiments. It is a weak acid, which can lose Hydrogen ion, H+ ions in solution. The nonionized phenolphthalein molecule is colorless and the double deprotonated phenolphthalein ion is Fuchsia (color), fuchsia. Further proton loss in higher pH occurs slowly and leads to a colorless form. Phenolphthalein ion in concentrated sulfuric acid is orange red due to sulfonation. Uses pH indicator Phenolphthalein's common use is as an indicator in acid-ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Analyst (journal)
''Analyst'' is a biweekly peer-reviewed scientific journal covering all aspects of analytical chemistry, bioanalysis, and detection science. It is published by the Royal Society of Chemistry and the editor-in-chief is Melanie Bailey (University of Surrey). Retrieved on 2023-05-29. The journal was established in 1877 by the Society for Analytical Chemistry. Abstracting and indexing The journal is abstracted and indexed in and |