|
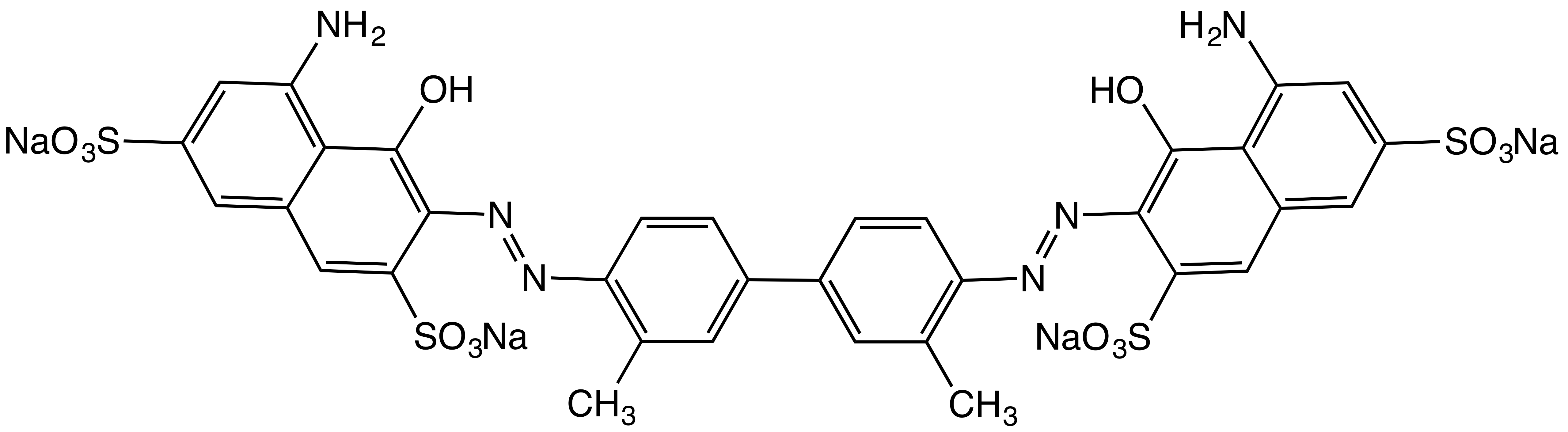
Sudan II
Sudan II (Solvent Orange 7, C.I. 12140, C18H16N2O) is a lysochrome (fat-soluble dye) azo dye used for staining of triglycerides in frozen sections, and some protein bound lipids and lipoproteins on paraffin sections. It has the appearance of red powder with melting point 156–158 °C and maximum absorption at 493(420) nm. Uses In industry, it is used to color nonpolar substances like oils, fats, waxes, greases, various hydrocarbon products, and acrylic emulsion An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althou ...s. It was used as food dye under the designation FD&C Red 32 in the US until the FDA banned its use in food in 1956 due to toxicity. References {{Stains Azo dyes Sudan dyes 2-Naphthols Alkyl-substituted benzenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysochrome
A lysochrome is a soluble dye used for histochemical staining of lipids, which include triglycerides, fatty acids, and lipoproteins. Lysochromes such as Sudan IV Sudan IV (C24H20N4O) is a lysochrome (fat-soluble dye) diazo dye used for the staining of lipids, triglycerides and lipoproteins on frozen paraffin sections. It has the appearance of reddish brown crystals with melting point 199 °C and max ... dissolve in the lipid and show up as colored regions. The dye does not stick to any other substrates, so a quantification or qualification of lipid presence can be obtained. The name was coined by John Baker (biologist) in his book "Principles of Biological Microtechnique", published in 1958, from the Greek words lysis (solution) and chroma (colour).Baker, J.R. 1958. Principles of Biological Microtechnique. London: Methuen, p.297-298. References {{Reflist Biochemistry methods Lipids Histochemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group, but some dyes called "disazo dyes" contain two azo groups, some dyes called "trisazo dyes" contain three azo groups and are or more. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staining (biology)
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific ( DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are used for view ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triglycerides
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and '' glyceride''). Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils. Many types of triglycerides exist. One specific classification focuses on saturated and unsaturated types. Saturated fats have ''no'' C=C groups; unsaturated fats feature one or more C=C groups. Unsaturated fats tend to have a lower melting point than saturated analogues; as a result, they are often liquid at room temperature. Chemical structure Triglycerides are tri-esters consisting of a glycerol bound to three fatty acid molecules. Alcohols have a hydroxyl (HO–) group. Organic acids have a carboxyl (–COOH) group. Alcohols and orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/ unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipoproteins
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid (also known as fat) molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are lipoproteins. Examples include plasma lipoprotein particles ( HDL, LDL, IDL, VLDL and chylomicrons). Subgroups of these plasma particles are primary drivers or modulators of atherosclerosis. Scope Transmembrane lipoproteins Some transmembrane proteolipids, especially those found in bacteria, are refer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paraffin Wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to melt above approximately , and its boiling point is above . Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons. It is distinct from kerosene and other petroleum products that are sometimes called paraffin. Un-dyed, unscented paraffin candles are odorless and bluish-white. Paraffin wax was first created by Carl Reichenbach in Germany in 1830 and marked a major advancement in candlemaking technology, as it burned more cleanly and reliably than tallow candles and was cheaper to produce. In chemistry, ''paraffin'' is used synonymously with ''alkane'', indicating hydrocarbons with the general formula C''n''H2''n''+2. The name is derived from Latin ''par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grease (lubricant)
Grease is a solid or semisolid lubricant formed as a dispersion of thickening agents in a liquid lubricant. Grease generally consists of a soap emulsified with mineral or vegetable oil. A common feature of greases is that they possess a high initial viscosity, which upon the application of shear, drops to give the effect of an oil-lubricated bearing of approximately the same viscosity as the base oil used in the grease. This change in viscosity is called shear thinning. Grease is sometimes used to describe lubricating materials that are simply soft solids or high viscosity liquids, but these materials do not exhibit the shear-thinning properties characteristic of the classical grease. For example, petroleum jellies such as Vaseline are not generally classified as greases. Greases are applied to mechanisms that can be lubricated only infrequently and where a lubricating oil would not stay in position. They also act as sealants to prevent ingress of water and incompressible mater ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms ''colloid'' and ''emulsion'' are sometimes used interchangeably, ''emulsion'' should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid (the dispersed phase) is dispersed in the other (the continuous phase). Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working. Two liquids can form different types of emulsions. As an example, oil and water can form, first, an oil-in-water emulsion, in which the oil is the dispersed phase, and water is the continuous phase. Second, they can form a water-in-oil emulsion, in which water is the dispersed phase and oil is the continuous phase. Multiple emulsions are al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group, but some dyes called "disazo dyes" contain two azo groups, some dyes called "trisazo dyes" contain three azo groups and are or more. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |