|
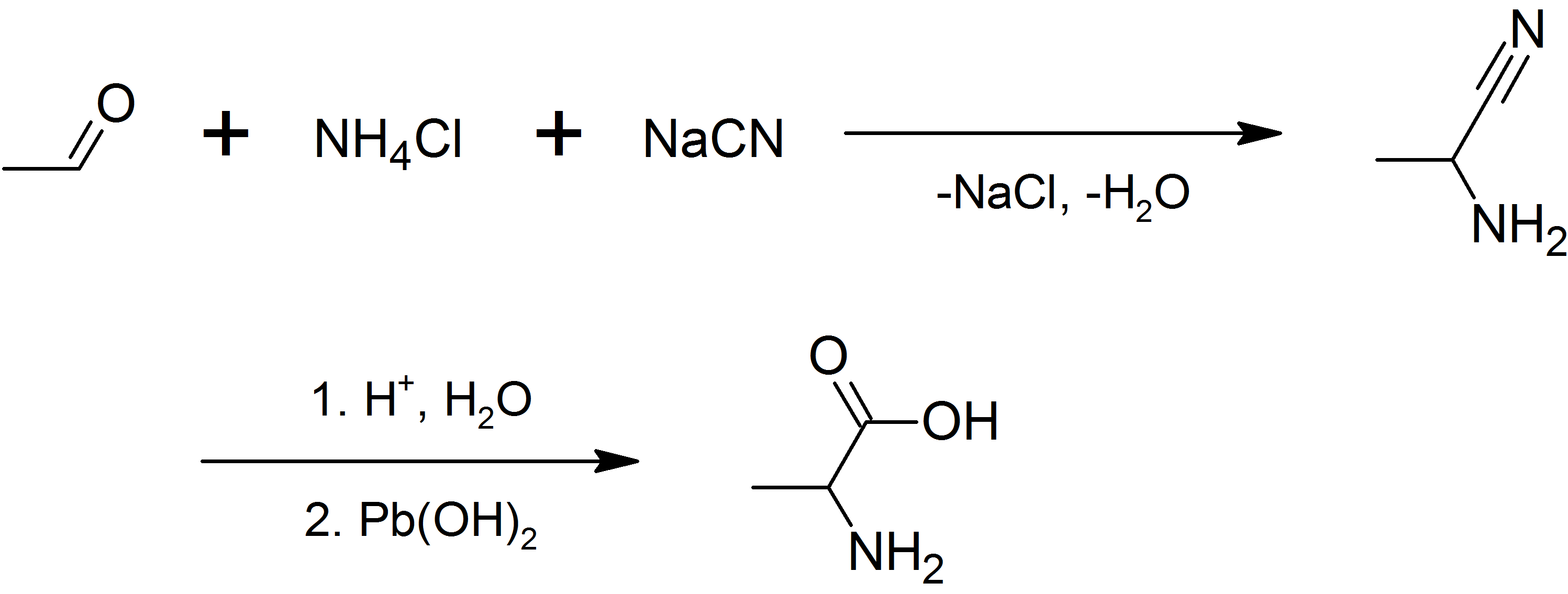
Stickland Fermentation
Stickland fermentation or The Stickland Reaction is the name for a chemical reaction that involves the coupled oxidation and reduction of amino acids to organic acids. The electron donor amino acid is oxidised to a volatile carboxylic acid one carbon atom shorter than the original amino acid. For example, alanine with a three carbon chain is converted to acetate with two carbons. The electron acceptor amino acid is reduced to a volatile carboxylic acid the same length as the original amino acid. For example, glycine with two carbons is converted to acetate. In this way, amino acid fermenting microbes can avoid using hydrogen ions as electron acceptors to produce hydrogen gas. Amino acids can be Stickland acceptors, Stickland donors, or act as both donor and acceptor. Only histidine cannot be fermented by Stickland reactions, and is oxidised. With a typical amino acid mix, there is a 10% shortfall in Stickland acceptors, which results in hydrogen production. Under very l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stickland Reaction General
Stickland is a surname of British origin, which may be a locational surname, indicating a person from the village of Stickland in the parish of Winterborne Stickland, Dorset. Alternatively, it may be a topographic name for a person who lived by a steep slope, from the Middle English ''stickel'' ("steep") and "land".''Dictionary of American Family Names''"Stickland Family History" Oxford University Press, 2013. Retrieved on 17 January 2016. The surname may refer to: *Jonathan Stickland (born 1983), American politician * L. H. Stickland, British biochemist *Lee Stickland, New Zealand soccer player * Paul Stickland (born 1957), British illustrator See also *Strickland (surname) Strickland is an English toponymic surname derived from the manor of Strickland in the historical county of Westmorland, now Cumbria, England, represented geographically by the modern villages of Great Strickland and Little Strickland. The surnam ... References {{surname Surnames of British Isles origin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stickland Fermentation English
Stickland is a surname of British origin, which may be a locational surname, indicating a person from the village of Stickland in the parish of Winterborne Stickland, Dorset. Alternatively, it may be a topographic name for a person who lived by a steep slope, from the Middle English ''stickel'' ("steep") and "land".''Dictionary of American Family Names''"Stickland Family History" Oxford University Press, 2013. Retrieved on 17 January 2016. The surname may refer to: *Jonathan Stickland (born 1983), American politician * L. H. Stickland, British biochemist * Lee Stickland, New Zealand soccer player * Paul Stickland (born 1957), British illustrator See also *Strickland (surname) Strickland is an English toponymic surname derived from the manor of Strickland in the historical county of Westmorland, now Cumbria, England, represented geographically by the modern villages of Great Strickland and Little Strickland. The surna ... References {{surname Surnames of British Isles origin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. Typical reducing agents undergo permanent chemical alteration through covalent or ionic reaction chemistry. This results in the complete and irreversible transfer of one or more electrons. In many chemical circumstances, however, the transfer of electronic charge to an electron acceptor may be only fractional, meaning an electron is not completely transferred, but results in an electron resonance between the donor and acceptor. This leads to the formation of charge transfer complexes in which the components largely retain their chemical identities. The electron donating power of a donor molecule is measured by its ionization potential which is the energy required to remove an electron from the highest occupied molecular orbital ( HOMO). The overall energy balance (ΔE), i.e., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common building block for biosynthesis. Nomenclature and common formula When part of a salt, the formula of the acetate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcosine
Sarcosine, also known as ''N''-methylglycine, or monomethylglycine, is a monopeptide with the formula CH3N(H)CH2CO2H. It exists at neutral pH as the zwitterion CH3N+(H)2CH2CO2−, which can be obtained as a white, water-soluble powder. Like some amino acids, sarcosine converts to a cation at low pH and an anion at high pH, with the respective formulas CH3N+(H)2CH2CO2H and CH3N(H)CH2CO2−. Sarcosine is a close relative of glycine, with a secondary amine in place of the primary amine. Sarcosine is ubiquitous in biological materials. It is used in manufacturing biodegradable surfactants and toothpastes as well as in other applications. Sarcosine is sweet to the taste. Biochemistry Sarcosine is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while glycine-''N''-methyl transferase generates sarcosine from glycine. Sarcosine is an amino acid derivative that is naturally found in musc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |