|
Spar (mineralogy)
Spar is an old mining or mineralogy term used to refer to crystals that have readily discernible faces. A spar will easily break or cleave into rhomboidal, cubical, or laminated fragments with smooth shiny surfaces. The various spar minerals were a historical term among miners and alchemists for any nonmetallic mineral akin to gypsum, known in Old English as spærstān, ''spear stone'', referring to its crystalline projections. Thus, the word spar in mineralogy has the same root as "spear,". Amongst miners the term "spar" today is frequently used alone to express any bright crystalline substance. Most frequently, spar describes easily cleaved, lightly colored nonmetallic minerals such as feldspar, calcite or baryte. Baryte ( Ba S O4), the main source of barium, is also called "heavy spar" ( Greek "barys" means "heavy"). Calcite often forms the dogtooth spar crystals found in vugs and caves. Formation underwater Generally, a spar will form underwater, either in a phreat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dogtooth Spar
Dogtooth spar is a speleothem that consists of large calcite crystals that form through mineral precipitation of water-borne calcite. Dogtooth spar crystals are found in caves, open spaces including veins and fractures, and geodes. They are so named for their resemblance to dog's teeth. The crystals are generally centimeters long, but anomalous samples decimeters long exist, notably in Sitting Bull Crystal Caverns. A layer of crystalline calcite can be found underneath the surface of crystal points. The crystals typically consist of acute scalenohedrons, twelve triangular crystal faces that ideally form scalene triangles. However, modification of these faces is common, and some may have many more than three edges. Calcite crystallizes in the rhombohedral system, and the most common scalenohedron form has the Miller index Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices. In particular, a family of lattice planes o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystals
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iceland Spar
Iceland spar, formerly called Iceland crystal ( , ) and also called optical calcite, is a transparent variety of calcite, or crystallized calcium carbonate, originally brought from Iceland, and used in demonstrating the polarization of light. Formation and composition Iceland spar is a colourless, transparent variety of calcium carbonate (CaCO3). It crystallizes in the trigonal system, typically forming rhombohedral crystals.Hughes, H. Herbert., Iceland spar and optical fluorite: ''U. S. Bureau of Mines, Information Circular'' 6468 (1931) It has a Mohs hardness of 3 and exhibits double refraction, splitting a ray of light into two rays that travel at different speeds and directions. Iceland spar forms in sedimentary environments, mainly limestone and dolomite rocks, but it also forms in hydrothermal veins and evaporite deposits. It precipitates from solutions rich in calcium and carbonate ions, influenced by temperature, pressure, and impurities. The most common crystal stru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenite (mineral)
Selenite, satin spar, desert rose, and gypsum flower are crystal habit varieties of the mineral gypsum. All varieties of gypsum, including selenite and alabaster, are composed of calcium sulfate dihydrate (meaning that it has two molecules of water), with the chemical formula CaSO4·2H2O. Selenite contains no selenium; the similar names both derive from Greek ( 'Moon'). Some of the largest crystals ever found are of selenite, the largest specimen found in the Naica Mine's Cave of the Crystals being 12 meters long and weighing 12 tons. History and etymology "Selenite" is mostly synonymous with gypsum, but from the 15th century, it has named the transparent variety that occurs in crystals or crystalline masses. The name derives through Middle English from Latin , ultimately from Greek (, ). It got this name because people historically believed the mineral waxed and waned with the cycles of the Moon. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution (chemistry)
In chemistry, a solution is defined by IUPAC as "A liquid or solid phase containing more than one substance, when for convenience one (or more) substance, which is called the solvent, is treated differently from the other substances, which are called solutes. When, as is often but not necessarily the case, the sum of the mole fractions of solutes is small compared with unity, the solution is called a dilute solution. A superscript attached to the ∞ symbol for a property of a solution denotes the property in the limit of infinite dilution." One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term " aqueous solution" is used when one of the solvents is water. Types ''Homogeneous'' means that the components of the mixture form a single phase. ''Heterogeneous'' means that the components of the mixture are of different phase. The properties of the mixture (such as concentration, temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
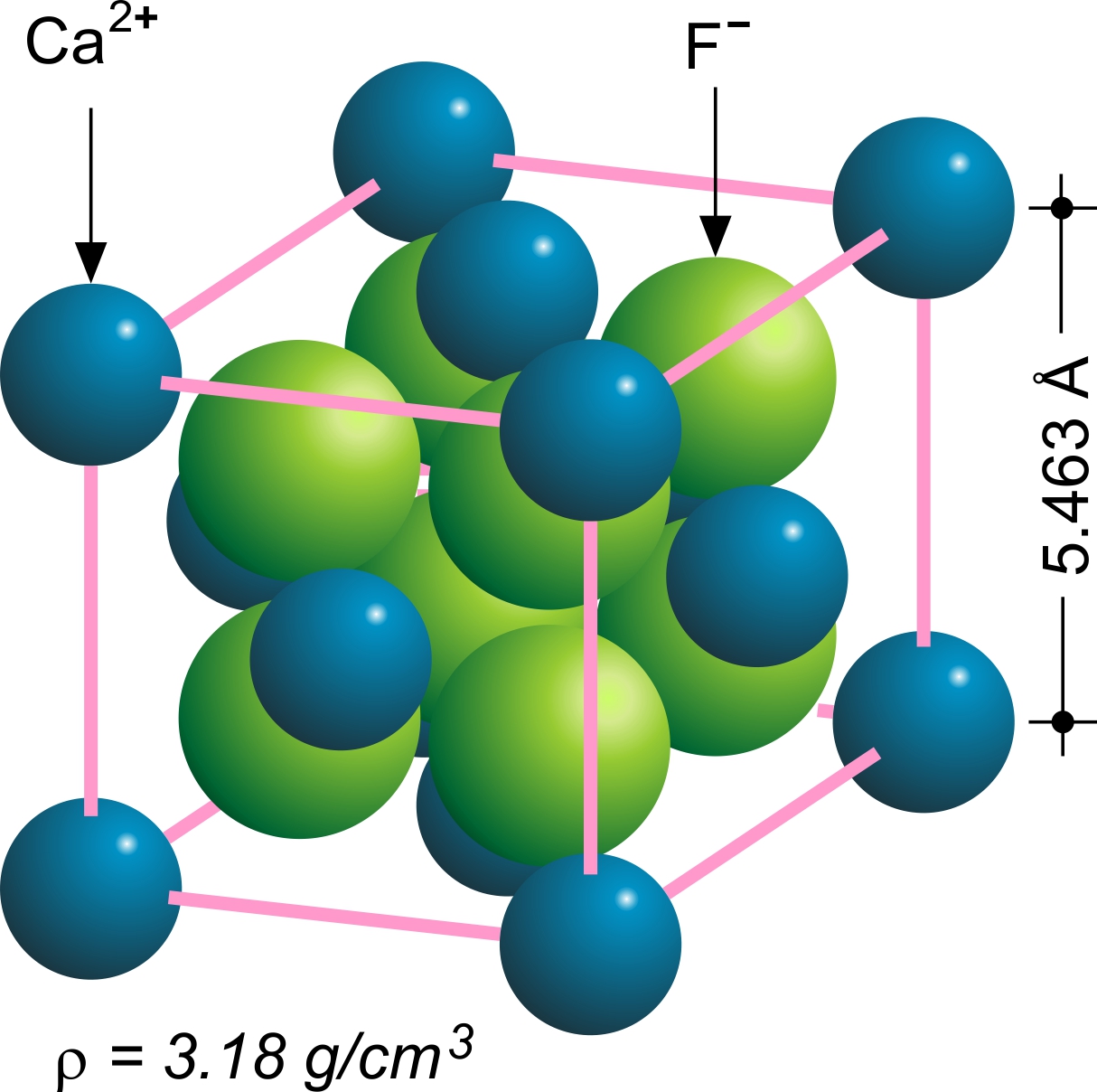
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quartz
Quartz is a hard, crystalline mineral composed of silica (silicon dioxide). The Atom, atoms are linked in a continuous framework of SiO4 silicon–oxygen Tetrahedral molecular geometry, tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical formula of Silicon dioxide, SiO2. Quartz is, therefore, classified structurally as a Silicate mineral#Tectosilicates, framework silicate mineral and compositionally as an oxide mineral. Quartz is the second most abundant mineral in Earth's continental crust, behind feldspar. Quartz exists in two forms, the normal α-quartz and the high-temperature β-quartz, both of which are chiral. The transformation from α-quartz to β-quartz takes place abruptly at . Since the transformation is accompanied by a significant change in volume, it can easily induce microfracturing of ceramics or rocks passing through this temperature threshold. There are many different varieties of quartz, several of which are classifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halite
Halite ( ), commonly known as rock salt, is a type of salt, the mineral (natural) form of sodium chloride ( Na Cl). Halite forms isometric crystals. The mineral is typically colorless or white, but may also be light blue, dark blue, purple, pink, red, orange, yellow or gray depending on inclusion of other materials, impurities, and structural or isotopic abnormalities in the crystals. It commonly occurs with other evaporite deposit minerals such as several of the sulfates, halides, and borates. The name ''halite'' is derived from the Ancient Greek word for "salt", ἅλς (''háls''). Occurrence Halite dominantly occurs within sedimentary rocks where it has formed from the evaporation of seawater or salty lake water. Vast beds of sedimentary evaporite minerals, including halite, can result from the drying up of enclosed lakes and restricted seas. Such salt beds may be hundreds of meters thick and underlie broad areas. Halite occurs at the surface today in playas in reg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bottlebrushes (Cave Formation)
A bottlebrush is a cave formation which results when a stalactite is immersed in rising water which is supersaturated with calcium carbonate Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel .... The stalactite becomes coated with pool spar. References Speleothems {{geology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalactite
A stalactite (, ; , ) is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension (chemistry), suspension, or is capable of being melting, melted, may form a stalactite. Stalactites may be composed of lava, minerals, mud, peat, pitch (resin), pitch, sand, Geyserite, sinter, and amberat (crystallized urine of pack rats). A stalactite is not necessarily a speleothem, though speleothems are the most common form of stalactite because of the abundance of limestone caves. The corresponding formation on the floor of the cave is known as a stalagmite. Formation and type Limestone stalactites The most common stalactites are speleothems, which occur in limestone caves. They form through Deposition (geology), deposition of calcium carbonate and other minerals, which is precipitated from mineralized water Solution (chemistry), solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Table
The water table is the upper surface of the phreatic zone or zone of saturation. The zone of saturation is where the pores and fractures of the ground are saturated with groundwater, which may be fresh, saline, or brackish, depending on the locality. It can also be simply explained as the depth below which the ground is saturated. The portion above the water table is the vadose zone. It may be visualized as the "surface" of the subsurface materials that are saturated with groundwater in a given vicinity. In coarse soils, the water table settles at the surface where the water Hydraulic head, pressure head is equal to the atmospheric pressure (where gauge pressure = 0). In soils where capillary action is strong, the water table is pulled upward, forming a capillary fringe. The groundwater may be from precipitation or from more distant groundwater flowing into the aquifer. In areas with sufficient precipitation, water infiltrates through pore spaces in the soil, passing through t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |