|
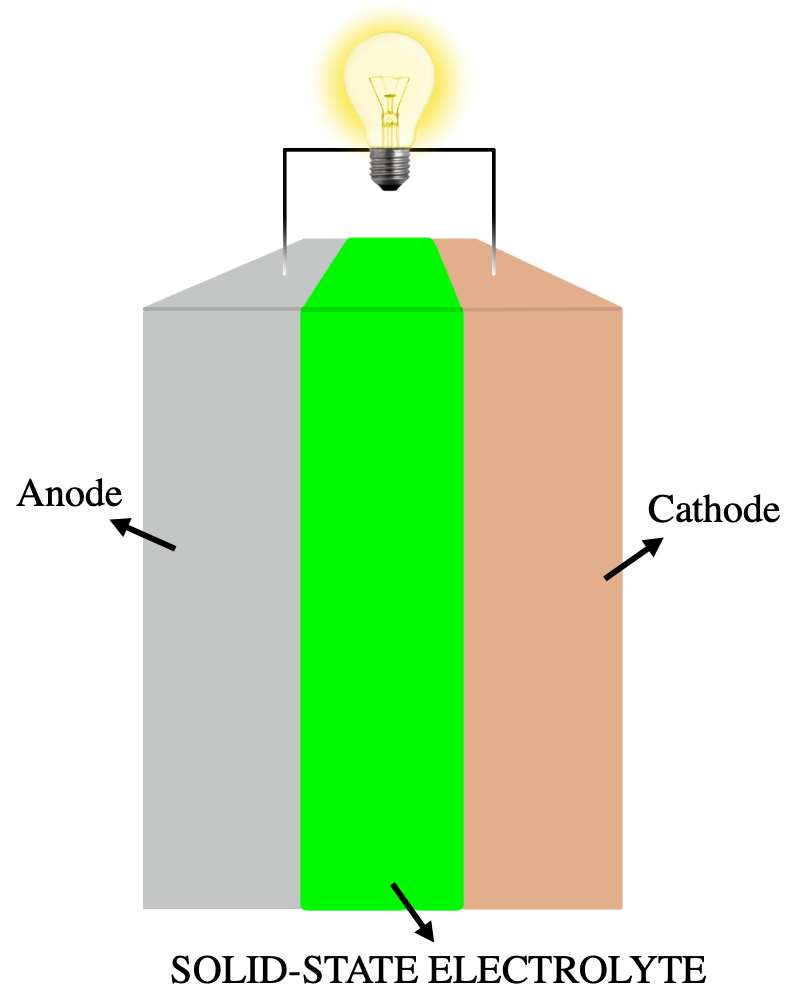
Solid-state Electrolyte
A solid-state electrolyte (SSE) is a solid Ionic conductivity (solid state), ionic conductor and electron-insulating electrolyte, material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage in substitution of the liquid electrolytes found in particular in the lithium-ion battery. Their main advantages are their absolute safety, no issues of leakages of toxic organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium Dendrite (crystal), dendrite suppression in the presence of a solid-state electrolyte membrane. The use of a high-capacity and low reduction potential anode, like lithium with a specific capacity of 386 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samsung
Samsung Group (; stylised as SΛMSUNG) is a South Korean Multinational corporation, multinational manufacturing Conglomerate (company), conglomerate headquartered in the Samsung Town office complex in Seoul. The group consists of numerous affiliated businesses, most of which operate under the Samsung brand, and is the largest (business conglomerate) in South Korea. Samsung has the world's List of most valuable brands, fifth-highest brand value. Founded in 1938 by Lee Byung-chul as a trading company, Samsung diversified into various sectors, including food processing, textiles, insurance, securities, and retail, over the next three decades. In the late 1960s, Samsung entered the electronics industry, followed by the construction and shipbuilding sectors in the mid-1970s—areas that would fuel its future growth. After Lee died in 1987, Samsung was divided into five business groups: Samsung Group, Shinsegae Group, CJ Group, Hansol Group, and JoongAng Ilbo, JoongAng Group. K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and " rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conductivity (electrolytic)
Conductivity or specific conductance of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter (S/m). Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems. In many cases, conductivity is linked directly to the total dissolved solids (TDS). High-quality deionized water has a conductivity of : \kappa = 0.05501 \pm 0.0001 μS/cm at 25 °C. This corresponds to a specific resistivity of : \rho = 18.18 \pm 0.03 MΩ⋅cm. The preparation of salt solutions often takes place in unsealed beakers. In this case the conductivity of purified water often is 10 to 20 times higher. A discussion can be found below. Typical drinking water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Voltammetry
In electrochemistry, cyclic voltammetry (CV) is a type of voltammetric measurement where the potential of the working electrode is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles in potential are repeated until the voltammetric trace reaches a cyclic steady state. The current at the working electrode is plotted versus the voltage at the working electrode to yield the cyclic voltammogram (see Figure 1). Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode. Experimental method In cyclic voltammetry (CV), the electrode potential is ramped linearly versus time in cyclical phases. The rate of voltage change over time during each of these phases is known as the scan rate (V/s). In a standar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Sweep Voltammetry
In analytical chemistry, linear sweep voltammetry is a method of voltammetry where the current at a working electrode is measured while the potential between the working electrode and a reference electrode is swept linearly in time. Oxidation or reduction of species is registered as a peak or trough in the current signal at the potential at which the species begins to be oxidized or reduced. Experimental method The experimental setup for linear sweep voltammetry utilizes a potentiostat and a three-electrode setup to deliver a potential to a solution and monitor its change in current. The three-electrode setup consists of a working electrode, an auxiliary electrode, and a reference electrode. The potentiostat delivers the potentials through the three-electrode setup. A potential, , is delivered through the working electrode. The slope of the potential vs. time graph is called the ''scan rate'' and can range from mV/s to 1,000,000 V/s. The working electrode is one of the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Window
The electrochemical window (EW) of a substance is the electrode electric potential range between which the substance is neither oxidized nor reduced. The EW is one of the most important characteristics to be identified for solvents and electrolytes used in electrochemical applications. The EW is a term that is commonly used to indicate the potential range and the potential difference. It is calculated by subtracting the reduction potential (cathodic limit) from the oxidation potential (anodic limit). When the substance of interest is water, it is often referred to as the ''water window''. This range is important for the efficiency of an electrode. Out of this range, the electrodes will react with the electrolyte, instead of driving the electrochemical reaction. In principle, ammonia has an extremely small electrochemical window, but thermodynamically-favored reactions less than 1 V outside the window are very slow. Consequently, the electrochemical window for many practi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tensile Test
Tensile testing, also known as tension testing, is a fundamental materials science and engineering test in which a sample is subjected to a controlled tension until failure. Properties that are directly measured via a tensile test are ultimate tensile strength, breaking strength, maximum elongation and reduction in area. From these measurements the following properties can also be determined: Young's modulus, Poisson's ratio, yield strength, and strain-hardening characteristics. ''Uniaxial tensile testing'' is the most commonly used for obtaining the mechanical characteristics of isotropic materials. Some materials use biaxial tensile testing. The main difference between these testing machines being how load is applied on the materials. Purposes of tensile testing Tensile testing might have a variety of purposes, such as: *Select a material or item for an application *Predict how a material will perform in use: normal and extreme forces. * Determine if, or verify that, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strength Of Materials
Strength may refer to: Personal trait *Physical strength, as in people or animals *Character strengths like those listed in the Values in Action Inventory *The exercise of willpower Physics * Mechanical strength, the ability to withstand an applied stress or load without structural failure ** Compressive strength, the capacity to withstand axially directed pushing forces **Tensile strength, the maximum stress while being stretched or pulled before necking ** Shear strength, the ability to withstand shearing * Strength (explosive), the ability of an explosive to move surrounding material * Field strength, the magnitude of a field's vector * Signal strength, in telecommunications *Strength (material), the behavior of solid objects subject to stresses and strains Music * Strength (American band), a band from Portland, Oregon * Strength (Japanese band), a band from Sendai, Miyagi, Japan * ''Strength'' (The Alarm album), 1985 * ''Strength'' (Enuff Z'nuff album), 1991 *''Stre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroanalytical Methods
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The three main categories are potentiometry (the difference in electrode potentials is measured), amperometry (electric current is the analytical signal), coulometry (charge passed during a certain time is recorded). Potentiometry Potentiometry passively measures the potential of a solution between two electrodes, affecting the solution very little in the process. One electrode is called the reference electrode and has a constant potential, while the other one is an indicator electrode whose potential changes with the sample's composition. Therefore, the difference in potential between the two electrodes gives an assessment of the sampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Transport Number
In chemistry, ion transport number, also called the transference number, is the fraction of the total electric current carried in an electrolyte by a given ionic species : :t_i = \frac Differences in transport number arise from differences in electrical mobility. For example, in an aqueous solution of sodium chloride, less than half of the current is carried by the positively charged sodium ions (cations) and more than half is carried by the negatively charged chloride ions (anions) because the chloride ions are able to move faster, i.e., chloride ions have higher mobility than sodium ions. The sum of the transport numbers for all of the ions in solution always equals unity: :\sum_i t_i = 1 The concept and measurement of transport number were introduced by Johann Wilhelm Hittorf in the year 1853. Liquid junction potential can arise from ions in a solution having different ion transport numbers. At zero concentration, the limiting ion transport numbers may be expressed in term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Impedance Spectroscopy
Dielectric spectroscopy (which falls in a subcategory of the impedance spectroscopy) measures the dielectric properties of a medium as a function of frequency.Kremer F., Schonhals A., Luck W. Broadband Dielectric Spectroscopy. – Springer-Verlag, 2002. It is based on the interaction of an external field with the electric dipole moment of the sample, often expressed by permittivity. It is also an experimental method of characterizing electrochemical systems. This technique measures the impedance of a system over a range of frequencies, and therefore the frequency response of the system, including the energy storage and dissipation properties, is revealed. Often, data obtained by electrochemical impedance spectroscopy (EIS) is expressed graphically in a Bode plot or a Nyquist plot. Impedance is the opposition to the flow of alternating current (AC) in a complex system. A passive complex electrical system comprises both energy dissipater (resistor) and energy storage (capacitor) e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |