Conductivity (electrolytic) on:
[Wikipedia]
[Google]
[Amazon]
Conductivity or specific conductance of an  In many cases, conductivity is linked directly to the
In many cases, conductivity is linked directly to the
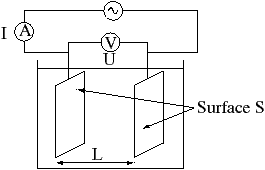 The
The
 In a typical experiment under the fume hood in an unsealed beaker the conductivity of purified water increases typically nonlinearly from values below 1 μS/cm to values close 3.5 μS/cm at 95 °C. This temperature dependence has to be taken into account particularly in dilute salt solutions.
In a typical experiment under the fume hood in an unsealed beaker the conductivity of purified water increases typically nonlinearly from values below 1 μS/cm to values close 3.5 μS/cm at 95 °C. This temperature dependence has to be taken into account particularly in dilute salt solutions.
Conductivity of concentrated solutions of electrolytes in methyl and ethyl alcohols
Concentrated solutions and ionic cloud model
H. L. Friedman, F. Franks, Aqueous Simple Electrolytes Solutions
{{Authority control Electrochemical concepts Physical chemistry Water quality indicators
electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens
Siemens AG ( ) is a German multinational technology conglomerate. It is focused on industrial automation, building automation, rail transport and health technology. Siemens is the largest engineering company in Europe, and holds the positi ...
per meter (S/m).
Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for hu ...
systems.
total dissolved solids
Total dissolved solids (TDS) is a measure of the dissolved solids, dissolved combined content of all inorganic compound, inorganic and organic compound, organic substances present in a liquid in molecule, molecular, ionized, or micro-granular (so ...
(TDS).
High-quality deionized water has a conductivity of
: μS/cm at 25 °C.
This corresponds to a specific resistivity of
: MΩ⋅cm.
The preparation of salt solutions often takes place in unsealed beakers. In this case the conductivity of purified water often is 10 to 20 times higher. A discussion can be found below.
Typical drinking water is in the range of 200–800 μS/cm, while sea water is about 50 mS/cm (or 0.05 S/cm).
Conductivity is traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's law of concentration dependence and additivity of ionic contributions. Lars Onsager
Lars Onsager (November 27, 1903 – October 5, 1976) was a Norwegian American physical chemist and theoretical physicist. He held the Gibbs Professorship of Theoretical Chemistry at Yale University. He was awarded the Nobel Prize in Chemist ...
gave a theoretical explanation of Kohlrausch's law by extending Debye–Hückel theory.
Units
The SI unit of conductivity is S/m, and unless otherwise qualified, it refers to 25 °C. More generally encountered is the traditional unit of μS/cm. The commonly used standard cell has a , and thus for very pure water in equilibrium with air would have a resistance of about 106 ohms, known as a megohm. Ultra-pure water could achieve 18 megohms or more. Thus in the past, megohm-cm was used, sometimes abbreviated to "megohm". Sometimes, conductivity is given in "microsiemens" (omitting the distance term in the unit). While this is an error, it can often be assumed to be equal to the traditional μS/cm. Often, by typographic limitations μS/cm is expressed as uS/cm. The conversion of conductivity (in μS/cm) to the total dissolved solids (in mg/kg) depends on the chemical composition of the sample and can vary between 0.54 and 0.96. Typically, the conversion is done assuming that the solid is sodium chloride; 1 μS/cm is then equivalent to about 0.64 mg of NaCl per kg of water. Molar conductivity has the SI unit S⋅m2⋅mol−1. Older publications use the unit Ω−1⋅cm2⋅mol−1.Measurement
 The
The electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
of a solution of an electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
is measured by determining the resistance of the solution between two flat or cylindrical electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s separated by a fixed distance. An alternating voltage is generally used in order to minimize water electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of c ...
. The resistance is measured by a conductivity meter. Typical frequencies used are in the range 1–3 kHz. The dependence on the frequency is usually small, but may become appreciable at very high frequencies, an effect known as the Debye–Falkenhagen effect.
A wide variety of instrumentation is commercially available. Most commonly, two types of electrode sensors are used: electrode-based sensors and inductive sensors. Electrode sensors with a static design are suitable for low and moderate conductivities and exist in various types, having either two or four electrodes, where electrodes can be arranged oppositely, flat or in a cylinder. Electrode cells with a flexible design, where the distance between two oppositely arranged electrodes can be varied, offer high accuracy and can also be used for the measurement of highly conductive media. Inductive sensors are suitable for harsh chemical conditions but require larger sample volumes than electrode sensors. Conductivity sensors are typically calibrated with KCl solutions of known conductivity. Electrolytic conductivity is highly temperature-dependent, but many commercial systems offer automatic temperature correction. Tables of reference conductivities are available for many common solutions.
Definitions
Resistance is proportional to the distance between the electrodes and is inversely proportional to the cross-sectional area of the sample (noted on the figure above). Writing (rho) for the specific resistance, orresistivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity i ...
,
:
In practice the conductivity cell is calibrated by using solutions of known specific resistance , so the individual quantities and need not be known precisely, but only their ratio. If the resistance of the calibration solution is , a cell constant, defined as the ratio of and ( = ), is derived:
:
The specific conductance (conductivity) (kappa) is the reciprocal of the specific resistance:
:
Sometimes the conductance (reciprocical of the resistance) is denoted as = . Then the specific conductance (kappa) is
:
Conductivity is also temperature-dependent.
Theory
The specific conductance of a solution containing one electrolyte depends on the concentration of the electrolyte. Therefore, it is convenient to divide the specific conductance by concentration. This quotient, termed molar conductivity, is denoted by : :Strong electrolytes
Strong electrolytes are hypothesized todissociate
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an aci ...
completely in solution. The conductivity of a solution of a strong electrolyte at low concentration follows Kohlrausch's Law:
:
where is known as the limiting molar conductivity, is an empirical constant, and is the electrolyte concentration. ("Limiting" here means "at the limit of the infinite dilution".) In effect, the observed conductivity of a strong electrolyte becomes directly proportional to concentration at sufficiently low concentrations, i.e. when
:
As the concentration is increased, however, the conductivity no longer rises in proportion.
Moreover, Kohlrausch also found that the limiting conductivity of an electrolyte,
: and ,
are the limiting molar conductivities of the individual ions.
The following table gives values for the limiting molar conductivities for some selected ions.
An interpretation of these results was based on the theory of Debye and Hückel, yielding the Debye–Hückel–Onsager theory:
:
where and are constants that depend only on known quantities such as temperature, the charges on the ions and the dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insul ...
and viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
of the solvent. As the name suggests, this is an extension of the Debye–Hückel theory, due to Onsager. It is very successful for solutions at low concentration.
Weak electrolytes
A weak electrolyte is one that is never fully dissociated (there is a mixture of ions and neutral molecules in equilibrium). In this case there is no limit of dilution below which the relationship between conductivity and concentration becomes linear. Instead, the solution becomes ever more fully dissociated at weaker concentrations, and for low concentrations of "well behaved" weak electrolytes, the degree of dissociation of the weak electrolyte becomes proportional to the inverse square root of the concentration. Typical weak electrolytes are weak acids and weak bases. The concentration of ions in a solution of a weak electrolyte is less than the concentration of the electrolyte itself. For acids and bases the concentrations can be calculated when the value or values of theacid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative property, quantitative measure of the acid strength, strength of an acid in Solution (chemistry), solution. I ...
are known.
For a monoprotic acid HA obeying the inverse square root law, with a dissociation constant , an explicit expression for the conductivity as a function of concentration , known as Ostwald's dilution law, can be obtained:
:
Various solvents exhibit the same dissociation if the ratio of relative permittivities equals the ratio cubic roots of concentrations of the electrolytes (Walden's rule).
Higher concentrations
Both Kohlrausch's law and the Debye–Hückel–Onsager equation break down as the concentration of the electrolyte increases above a certain value. The reason for this is that as concentration increases the average distance between cation and anion decreases, so that there is more interactions between close ions. Whether this constitutesion association
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each ...
is a moot point. However, it has often been assumed that cation and anion interact to form an ion pair. So an "ion-association" constant can be derived for the association equilibrium between ions A+ and B−:
: A+ + B− A+B−with = .
Davies describes the results of such calculations in great detail, but states that should not necessarily be thought of as a true equilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
, rather, the inclusion of an "ion-association" term is useful in extending the range of good agreement between theory and experimental conductivity data. Various attempts have been made to extend Onsager's treatment to more concentrated solutions.
The existence of a so-called ''conductance minimum'' in solvents having the relative permittivity
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the vacuum permittivity, electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric co ...
under 60 has proved to be a controversial subject as regards interpretation. Fuoss and Kraus suggested that it is caused by the formation of ion triplets, and this suggestion has received some support recently.
Other developments on this topic have been done by Theodore Shedlovsky, E. Pitts, R. M. Fuoss, Fuoss and Shedlovsky, Fuoss and Onsager.
Mixed solvents systems
The limiting equivalent conductivity of solutions based on mixed solvents like water alcohol has minima depending on the nature of alcohol. For methanol the minimum is at 15 molar % water, and for the ethanol at 6 molar % water.Conductivity versus temperature
Generally the conductivity of a solution increases with temperature, as the mobility of the ions increases. For comparison purposes reference values are reported at an agreed temperature, usually 298 K (≈ 25 °C or 77 °F), although occasionally 20 °C (68 °F) is used. So-called "compensated" measurements are made at a convenient temperature but the value reported is a calculated value of the expected value of conductivity of the solution, as if it had been measured at the reference temperature. Basic compensation is normally done by assuming a linear increase of conductivity versus temperature of typically 2% per kelvin. This value is broadly applicable for most salts at room temperature. Determination of the precise temperature coefficient for a specific solution is simple, and instruments are typically capable of applying the derived coefficient (i.e. other than 2%). Measurements of conductivity versus temperature can be used to determine theactivation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
, using the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 188 ...
:
where is the exponential prefactor, the gas constant, and the absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
in kelvin
The kelvin (symbol: K) is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature (absolute zero), taken to be 0 K. By de ...
s.
Solvent isotopic effect
The change in conductivity due to the isotope effect for deuterated electrolytes is sizable.Applications
Despite the difficulty of theoretical interpretation, measured conductivity is a good indicator of the presence or absence of conductive ions in solution, and measurements are used extensively in many industries. For example, conductivity measurements are used to monitor quality in public water supplies, in hospitals, in boiler water and industries that depend on water quality such as brewing. This type of measurement is not ion-specific; it can sometimes be used to determine the amount oftotal dissolved solids
Total dissolved solids (TDS) is a measure of the dissolved solids, dissolved combined content of all inorganic compound, inorganic and organic compound, organic substances present in a liquid in molecule, molecular, ionized, or micro-granular (so ...
(TDS) if the composition of the solution and its conductivity behavior are known. Conductivity measurements made to determine water purity will not respond to non conductive contaminants (many organic compounds fall into this category), therefore additional purity tests may be required depending on application.
Applications of TDS measurements are not limited to industrial use; many people use TDS as an indicator of the purity of their drinking water. Additionally, aquarium enthusiasts are concerned with TDS, both for freshwater and salt water aquariums. Many fish and invertebrates require quite narrow parameters for dissolved solids. Especially for successful breeding of some invertebrates normally kept in freshwater aquariums—snails and shrimp primarily—brackish water with higher TDS, specifically higher salinity, water is required. While the adults of a given species may thrive in freshwater, this is not always true for the young and some species will not breed at all in non-brackish water.
Sometimes, conductivity measurements are linked with other methods to increase the sensitivity of detection of specific types of ions. For example, in the boiler water technology, the boiler blowdown is continuously monitored for "cation conductivity", which is the conductivity of the water after it has been passed through a cation exchange resin. This is a sensitive method of monitoring anion impurities in the boiler water in the presence of excess cations (those of the alkalizing agent usually used for water treatment). The sensitivity of this method relies on the high mobility of H+ in comparison with the mobility of other cations or anions. Beyond cation conductivity, there are analytical instruments designed to measure Degas conductivity, where conductivity is measured after dissolved carbon dioxide has been removed from the sample, either through reboiling or dynamic degassing.
Conductivity detectors are commonly used with ion chromatography
Ion chromatography (or ion-exchange chromatography) is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of Charge (chemistry), charged molecule ...
.
Conductivity of purified water in electrochemical experiments
The electronic conductivity of purified distilled water in electrochemical laboratory settings at room temperature is often between 0.05 and 1 μS/cm. Environmental influences during the preparation of salt solutions as gas absorption due to storing the water in an unsealed beaker may immediately increase the conductivity from 0.055 μS/cm and lead to values between 0.5 and 1 μS/cm. When distilled water is heated during the preparation of salt solutions, the conductivity increases even without adding salt. This is often not taken into account.See also
*Einstein relation (kinetic theory)
In physics (specifically, the kinetic theory of gases), the Einstein relation is a previously unexpected connection revealed independently by William Sutherland in 1904, Albert Einstein in 1905, and by Marian Smoluchowski in 1906 in their works ...
* Born equation
The Born equation can be used for estimating the electrostatic component of Gibbs free energy of solvation of an ion. It is an electrostatic model that treats the solvent as a continuous dielectric medium (it is thus one member of a class of method ...
* Debye–Falkenhagen effect
* Law of dilution
* Ion transport number
* Ionic atmosphere
* Wien effect
* Conductimetric titration - methods to determine the equivalence point
References
Further reading
* Hans Falkenhagen, ''Theorie der Elektrolyte'', S. Hirzel Verlag, Leipzig, 1971 *Conductivity of concentrated solutions of electrolytes in methyl and ethyl alcohols
Concentrated solutions and ionic cloud model
H. L. Friedman, F. Franks, Aqueous Simple Electrolytes Solutions
{{Authority control Electrochemical concepts Physical chemistry Water quality indicators