|
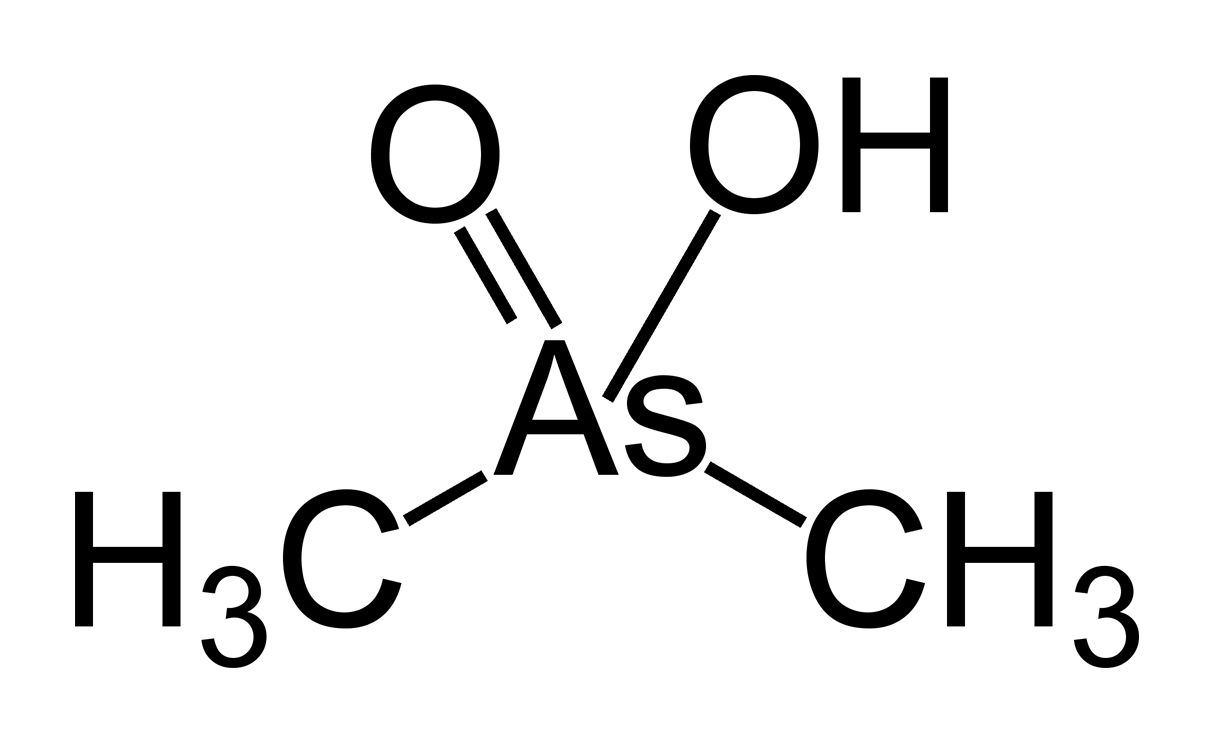
Sodium Cacodylate
Cacodylic acid is an organoarsenic compound with the formula (CH3)2 AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water. Neutralization of cacodylic acid with base gives cacodylate salts, e.g. sodium cacodylate. They are potent herbicides. Cacodylic acid/sodium cacodylate is a buffering agent in the preparation and fixation of biological samples for electron microscopy and in protein crystallography. History In the 18th century it was found that combining arsenic trioxide () and four equivalents of potassium acetate () gives a product called " Cadet's fuming liquid" which contains cacodyl oxide, and cacodyl, . Early research into "cacodyls" was reported by Robert Bunsen at the University of Marburg. Bunsen said of the compounds, "The smell of this body produces instantaneous tingling of the hands and feet, and even giddiness and insensibility... It is remarkable that when one is exposed to the sme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
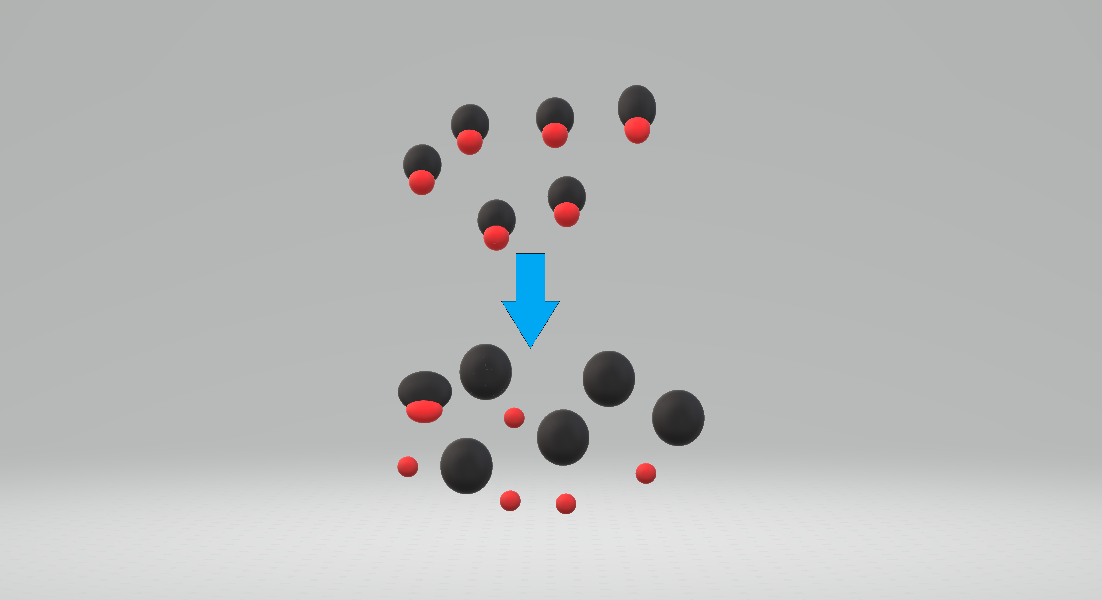
Ball-and-stick Model
In chemistry, the ball-and-stick model is a molecular model of a chemical substance which displays both the Molecular geometry, three-dimensional position of the atoms and the chemical bond, bonds between them. The atoms are typically represented by sphere (geometry), spheres, connected by rods which represent the bonds. Double bond, Double and triple bonds are usually represented by two or three curved rods, respectively, or alternately by correctly positioned sticks for the sigma bond, sigma and pi bonds. In a good model, the angles between the rods should be the same as the Bond angle, angles between the bonds, and the distances between the centers of the spheres should be proportional to the distances between the corresponding atomic nucleus, atomic nuclei. The chemical element of each atom is often indicated by the sphere's color. In a ball-and-stick model, the radius of the spheres is usually much smaller than the rod lengths, in order to provide a clearer view of the atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Acetate
Potassium acetate (also called potassium ethanoate), (CH3COOK) is the potassium salt of acetic acid. It is a hygroscopic solid at room temperature. Preparation It can be prepared by treating a potassium-containing base such as potassium hydroxide or potassium carbonate with acetic acid: :CH3COOH + KOH → CH3COOK + H2O This sort of reaction is known as an acid-base neutralization reaction. At saturation, the sesquihydrate in water solution (CH3COOK·1½H2O) begins to form semihydrate at 41.3 °C. Applications Deicing Potassium acetate (as a substitute for calcium chloride or magnesium chloride) can be used as a deicer to remove ice or prevent its formation. It offers the advantage of being less aggressive on soils and much less corrosive: for this reason, it is preferred for airport runways although it is more expensive. Fire extinguishing Potassium acetate is the extinguishing agent used in Class K fire extinguishers because of its ability to cool and form a crust o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ingestion
Ingestion is the consumption of a substance by an organism. In animals, it normally is accomplished by taking in a substance through the mouth into the gastrointestinal tract, such as through eating or drinking. In single-celled organisms, ingestion takes place by absorbing a substance through the cell membrane. Besides nutritional items, substances that may be ingested include medication (where ingestion is termed oral administration), recreational drugs, and substances considered inedible, such as foreign bodies or excrement. Ingestion is a common route taken by pathogenic organisms and poisons entering the body. Ingestion can also refer to a mechanism picking up something and making it enter an internal hollow of that mechanism, e.g. "''a grille was fitted to prevent the pump from ingesting driftwood''". Pathogens Some pathogens are transmitted via ingestion, including viruses, bacteria, and parasites. Most commonly, this takes place via the faecal-oral route. An interm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is toxic, corrosive, and flammable. Trace amounts in ambient atmosphere have a characteristic foul odor of rotten eggs. Swedish chemist Carl Wilhelm Scheele is credited with having discovered the chemical composition of purified hydrogen sulfide in 1777. Hydrogen sulfide is toxic to humans and most other animals by inhibiting cellular respiration in a manner similar to hydrogen cyanide. When it is inhaled or its salts are ingested in high amounts, damage to organs occurs rapidly with symptoms ranging from breathing difficulties to convulsions and death. Despite this, the human body produces small amounts of this sulfide and its mineral salts, and uses it as a signalling molecule. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen, such as in swamps and sewers; this process is commonly known as anaerobic digestio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Weak Acid
Acid strength is the tendency of an acid, symbolised by the chemical formula , to dissociate into a proton, , and an anion, . The dissociation or ionization of a strong acid in solution is effectively complete, except in its most concentrated solutions. : Examples of strong acids are hydrochloric acid (), perchloric acid (), nitric acid () and sulfuric acid (). A weak acid is only partially dissociated, or is partly ionized in water with both the undissociated acid and its dissociation products being present, in solution, in equilibrium with each other. : Acetic acid () is an example of a weak acid. The strength of a weak acid is quantified by its acid dissociation constant, K_a value. The strength of a weak organic acid may depend on substituent effects. The strength of an inorganic acid is dependent on the oxidation state for the atom to which the proton may be attached. Acid strength is solvent-dependent. For example, hydrogen chloride is a strong acid in aqueous solu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Defoliant
A defoliant is any herbicidal chemical sprayed or dusted on plants to cause their leaves to fall off. Defoliants are widely used for the selective removal of weeds in managing croplands and lawns. Worldwide use of defoliants, along with the development of other herbicides and pesticides, allowed for the Green Revolution, an increase in agricultural production in mid-20th century. Defoliants have also been used in warfare as a means to deprive an enemy of food crops and/or hiding cover, most notably by the United Kingdom during the Malayan Emergency and the United States in the Vietnam War. Defoliants were also used by Indonesian forces in various internal security operations. Use and application A primary application of defoliants is the selective killing of plants. Two of the oldest chemical herbicides used as defoliants are 2,4-Dichlorophenoxyacetic acid (2,4-D) and 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T). 2,4-D and 2,4,5-T are absorbed by broad-leafed plants, killin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vietnam War
The Vietnam War (1 November 1955 – 30 April 1975) was an armed conflict in Vietnam, Laos, and Cambodia fought between North Vietnam (Democratic Republic of Vietnam) and South Vietnam (Republic of Vietnam) and their allies. North Vietnam was supported by the Soviet Union and China, while South Vietnam was supported by the United States and other anti-communist nations. The conflict was the second of the Indochina wars and a proxy war of the Cold War between the Soviet Union and US. The Vietnam War was one of the postcolonial wars of national liberation, a theater in the Cold War, and a civil war, with civil warfare a defining feature from the outset. Direct United States in the Vietnam War, US military involvement escalated from 1965 until its withdrawal in 1973. The fighting spilled into the Laotian Civil War, Laotian and Cambodian Civil Wars, which ended with all three countries becoming Communism, communist in 1975. After the defeat of the French Union in the First Indoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agent Blue
Agent Blue is one of the " rainbow herbicides" that is known for use by the United States during the Vietnam War. It contained a mixture of dimethylarsinic acid (also known as cacodylic acid) and its related salt, sodium cacodylate, and water. History Largely inspired by the British use of herbicides and defoliants during the Malayan Emergency, killing rice was a military strategy from the very start of U.S. military involvement in Vietnam. At first, U.S. soldiers attempted to blow up rice paddies and rice stocks, using mortars and hand grenades. But grains of rice were far more durable than they understood, and were not easily destroyed. Every grain that survived was a seed to be collected and planted again. A 1967 report to the International War Crimes Tribunal stated that "The soldiers discovered that rice is one of the most maddeningly difficult substances to destroy; using thermite metal grenades it is almost impossible to make it burn and, even if one succeeds in scatteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Compound
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide ( metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Marburg
The Philipps University of Marburg () is a public research university located in Marburg, Germany. It was founded in 1527 by Philip I, Landgrave of Hesse, which makes it one of Germany's oldest universities and the oldest still operating Protestant university in the world. It is now a public university of the state of Hesse, without religious affiliation. The University of Marburg has about 23,500 students and 7,500 employees and is located in Marburg, a town of 76,000 inhabitants, with university buildings dotted in or around the town centre. About 14% of the students are international, the highest percentage in Hesse. It offers an international summer university programme and offers student exchanges through the Erasmus programme. History In 1609, the University of Marburg established the world's first professorship in chemistry. In 2012 it opened the first German interactive chemistry museum, called '. Its experimental course programme is aimed at encouraging young people ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Bunsen
Robert Wilhelm Eberhard Bunsen (; 30 March 1811 – 16 August 1899) was a German chemist. He investigated emission spectra of heated elements, and discovered caesium (in 1860) and rubidium (in 1861) with the physicist Gustav Kirchhoff. The Bunsen–Kirchhoff Award for spectroscopy is named after Bunsen and Kirchhoff. Bunsen also developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organic arsenic chemistry. With his laboratory assistant Peter Desaga, he developed the Bunsen burner, an improvement on the laboratory burners then in use. Early life and education Bunsen was born in Göttingen, Germany, in 1811, in what is now the state of Lower Saxony in Germany. Bunsen was the youngest of four sons of the University of Göttingen's chief librarian and professor of modern philology, Christian Bunsen (1770–1837). After attending school in Holzminden, Bunsen matriculated at Göttingen in 1828 and studied chemistry wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |