|
Sanguinarine Structure V
Sanguinarine is a polycyclic quaternary alkaloid. It is extracted from some plants, including the bloodroot plant, from whose taxonomic name, ''Sanguinaria canadensis,'' its name is drawn; the Mexican prickly poppy (''Argemone mexicana''); ''Chelidonium majus;'' and ''Macleaya cordata.'' Toxicity Sanguinarine is a toxin that kills animal cells through its action on the Na+/K+-ATPase, Na+/K+-ATPase transmembrane protein. Epidemic dropsy is a disease that results from ingesting sanguinarine. If applied to the skin, sanguinarine may cause a massive scab of dead flesh where it killed the cells where it was applied, called an ''eschar''. For this reason, sanguinarine is termed an escharotic. It is said to be 2.5 times more toxic than dihydrosanguinarine. Alternative medicine Native Americans once used sanguinarine in the form of bloodroot as a medical remedy, believing it had curative properties as an emetic, respiratory aid, and for a variety of ailments. In Colonial America, sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
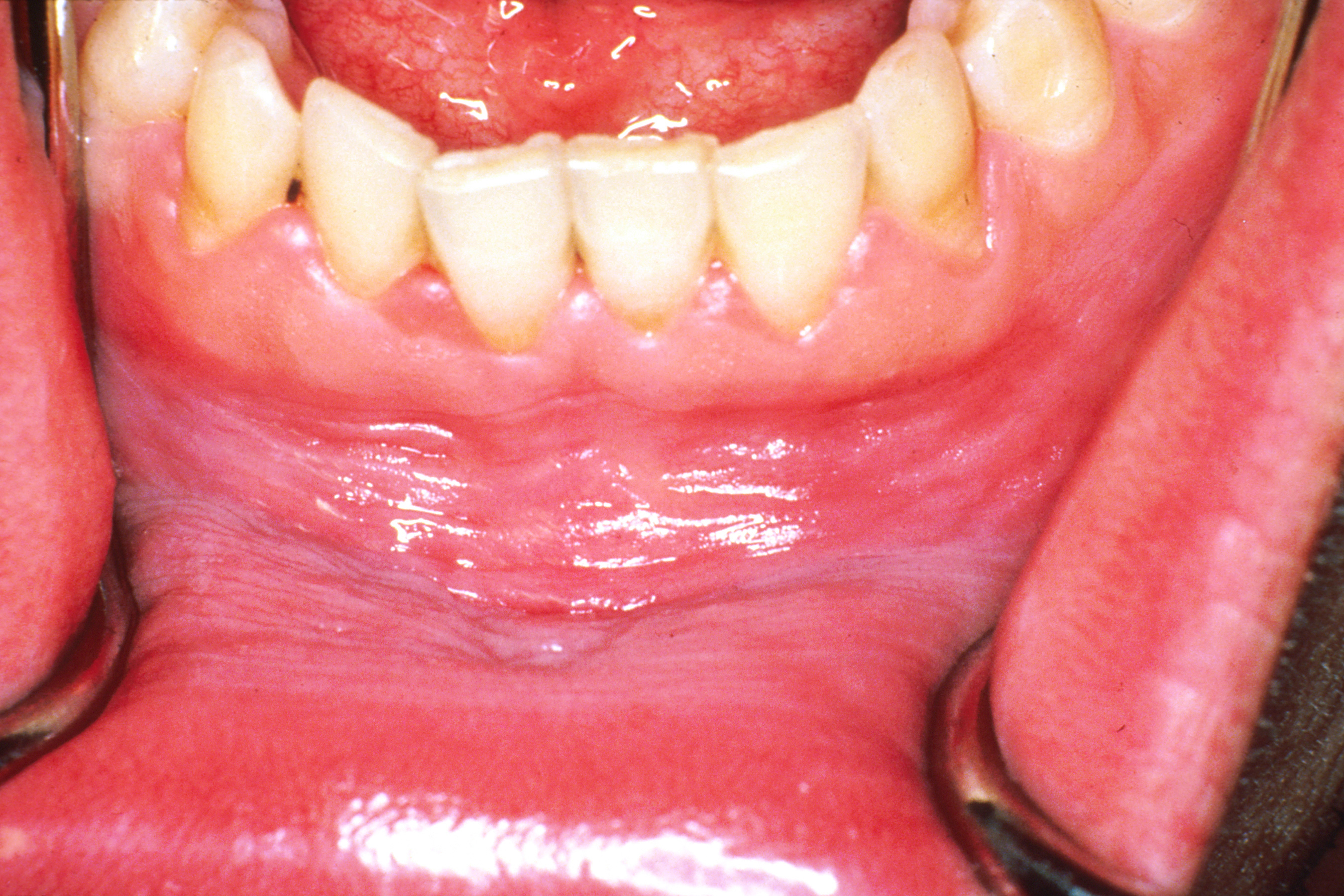
Oral Leukoplakia
Oral leukoplakia is a ''potentially malignant disorder'' affecting the oral mucosa. It is defined as "essentially an oral mucosal white lesion that cannot be considered as any other definable lesion." Oral leukoplakia is a white patch or plaque that develops in the oral cavity and is strongly associated with smoking. Leukoplakia is a firmly attached white patch on a mucous membrane which is associated with increased risk of cancer. The edges of the lesion are typically abrupt and the lesion changes with time. Advanced forms may develop red patches. There are generally no other symptoms. It usually occurs within the mouth, although sometimes mucosa in other parts of the gastrointestinal tract, urinary tract, or genitals may be affected. The cause of leukoplakia is unknown. Risk factors for formation inside the mouth include smoking, chewing tobacco, excessive alcohol, and use of betel nuts. One specific type is common in HIV/AIDS. It is a precancerous lesion, a tissue alteration in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Compounds
In chemistry, quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure , R being an alkyl group or an aryl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis Quaternary ammo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinoline Alkaloids
Quinoline alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from quinoline. Some quinoline alkaloids show antiseptic, convulsive or antineoplastic effects. Examples Alkaloids with a quinoline partial structure are widespread and are usually further subdivided according to their occurrence and biogenetic origin. Among the quinoline alkaloids are the cinchona alkaloids quinine and quinidine, which are important due to their therapeutic potential, furthermore cinchonine and cinchonidine, as well as some furoquinoline alkaloids and acridine alkaloids. Strychnine and brucine, alkaloids of the nux vomica, which have a hydrogenated quinoline system, are also counted among the quinoline alkaloids. Also nitramarine (1-(2-quinolinyl)-β-carboline) belongs to the quinoline alkaloids. File:Chinin.svg, Qinine File:Chinidin.svg, Qinidine File:Cinchonidin.svg, Cinchonidine File:Strychnine2.svg, Strychnine Occurrence The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoquinoline Alkaloids
Isoquinoline alkaloids are natural products of the group of alkaloids, which are chemically derived from isoquinoline. They form the largest group among the alkaloids. Isoquinoline alkaloids can be further classified based on their different chemical basic structures. The most common structural types are the benzylisoquinolines and the aporphines.Bettina Ruff: ''Chemische und biochemische Methoden zur stereoselektiven Synthese von komplexen Naturstoffen.'' Verlag Logos, Berlin 2012, , S. 8. () According to current knowledge, a total of about 2500 isoquinoline alkaloids are known nowadays, which are mainly formed by plants.Jennifer M. Finefield, David H. Sherman, Martin Kreitman, Robert M. Williams: ''Enantiomere Naturstoffe: Vorkommen und Biogenese.'' In: ''Angewandte Chemie.'' Wiley-VCH, Weinheim 2012, doi:10.1002/ange.201107204, S. 4905–4915. Known examples Morphin - Morphine.svg, Morphine Codein - Codeine.svg, Codeine Papaverin - Papaverine.svg, Papaverine Ber ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelidonine
Chelidonine is an isolate of '' Papaveraceae'' with acetylcholinesterase and butyrylcholinesterase inhibitory activity. Introduction Chelidonine is the major alkaloid component of '' Chelidonium majus''. ''Chelidonium majus L''. is the only species of the tribe Chelidonieae of the family Papaveraceae. Papaveraceae is rich in specific alkaloids. ''C. majus'' contains various isoquinoline alkaloids with protopine, protoberberine and benzophenanthridine structures. This benzophenanthridine alkaloid can induce apoptosis in some transformed or malignant cell lines. D-Chelidonine, the main alkaloid of ''Chelidonium majus'', was first isolated in 1839.Keller, K. (1971). Total synthesis of dl-chelidonine. ''Journal of the American Chemical Society'', ''117''(51), 3836. The supposed healing properties of greater celandine (''Chelidonium majus'') were believed in throughout Europe and Asia during the Imperial Roman period (Pliny 1966), and New World aboriginal cultures used BIA-conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berberine
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids found in such plants as '' Berberis vulgaris'' (barberry), '' Berberis aristata'' (tree turmeric), ''Mahonia aquifolium'' (Oregon grape), '' Hydrastis canadensis'' (goldenseal), '' Xanthorhiza simplicissima'' (yellowroot), ''Phellodendron amurense'' (Amur cork tree), '' Coptis chinensis'' (Chinese goldthread), '' Tinospora cordifolia'', ''Argemone mexicana'' (prickly poppy), and '' Eschscholzia californica'' (Californian poppy). Berberine is usually found in the roots, rhizomes, stems, and bark. Due to its yellow color, ''Berberis'' species were used to dye wool, leather, and wood. Under ultraviolet light, berberine shows a strong yellow fluorescence, making it useful in histology for staining heparin in mast cells. As a natural dye, berberine has a color index of 75160. Research and adverse effects The safety of using berberine for any condition is not adequately defined by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry Of Poisons Project Pic
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work (pharmacology), and how to collect D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrobenzophenanthridine Oxidase
Dihydrobenzophenanthridine oxidase (DHBP oxidase) is an enzyme. In the IUBMB Enzyme Nomenclature, dihydrobenzophenanthridine oxidase is . Dihydrobenzophenanthridine oxidase produces oxidized forms of benzophenanthridine alkaloids: * In ''Sanguinaria canadensis'' (bloodroot), dihydrobenzophenanthridine oxidase produces sanguinarine from dihydrosanguinarine, and chelirubine from dihydrochelirubine. * In ''Eschscholzia californica ''Eschscholzia californica'', the California poppy, golden poppy, California sunlight or cup of gold, is a species of flowering plant in the family Papaveraceae, native to the United States and Mexico. It is cultivated as an ornamental plant f ...'' (California poppy), dihydrobenzophenanthridine oxidase produces macarpine from dihydromacarpine. External links Chelirubine, Macarpine and Sanguinarine Biosynthesis* * * KEGG (Kyoto Encyclopedia of Genes and Genomes) Alkaloid biosynthesis I - Reference pathway EC 1.5.3 {{1.5-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Codeinone
Codeinone is 1/3 as active as codeine as an analgesic but it is an important intermediate in the production of hydrocodone, a painkiller about 3/4 the potency of morphine; as well as of oxycodone. The latter can also be synthesized from thebaine, however. Chemical structure Codeinone can be described as the methylether of morphinone: 3-methyl-morphinone. Codeinone can be also described as the ketone of codeine: codeine-6-one. Apoptotic activity Through renewed interest into possible anti-tumor activities of some of the opium alkaloid Alkaloids are a class of basic BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Th ...s and derivatives, unrelated to their antinociceptive properties and habit-forming effects, the oxidation product of codeine has been found to induce cell death in three different human cancer cell l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies ('' Papaver somniferum''). It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. There are numerous methods used to administer morphine: oral; sublingual; via inhalation; injection into a muscle; by injection under the skin; intravenously; injection into the space around the spinal cord; transdermal; or via rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during labor. Its maximum effect is reached after about 20 minutes when administered intravenously and 60 minutes when administered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reticuline
Reticuline is a chemical compound found in a variety of plants including '' Lindera aggregata'', ''Annona squamosa'', and ''Ocotea fasciculata'' (also known as ''Ocotea duckei''). It is based on the benzylisoquinoline structure. Reticuline is one of the alkaloids found in opium, and experiments in rodents suggest it possesses potent central nervous system depressing effects. It is the precursor of morphine and many other alkaloids. It is also toxic to dopaminergic neurons causing a form of atypical parkinsonism known as Guadeloupean Parkinsonism. Metabolism 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase uses ''S''-adenosyl methionine and 3'-hydroxy-''N''-methyl-(''S'')-coclaurine to produce ''S''-adenosylhomocysteine and (''S'')-reticuline. Reticuline oxidase uses (''S'')-reticuline and O2 to produce (''S'')- scoulerine and H2O2. Salutaridine synthase uses (''R'')-reticuline, NADPH, H+, and O2 to produce salutaridine, NADP+, and H2O. Salutaridine can then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |