|
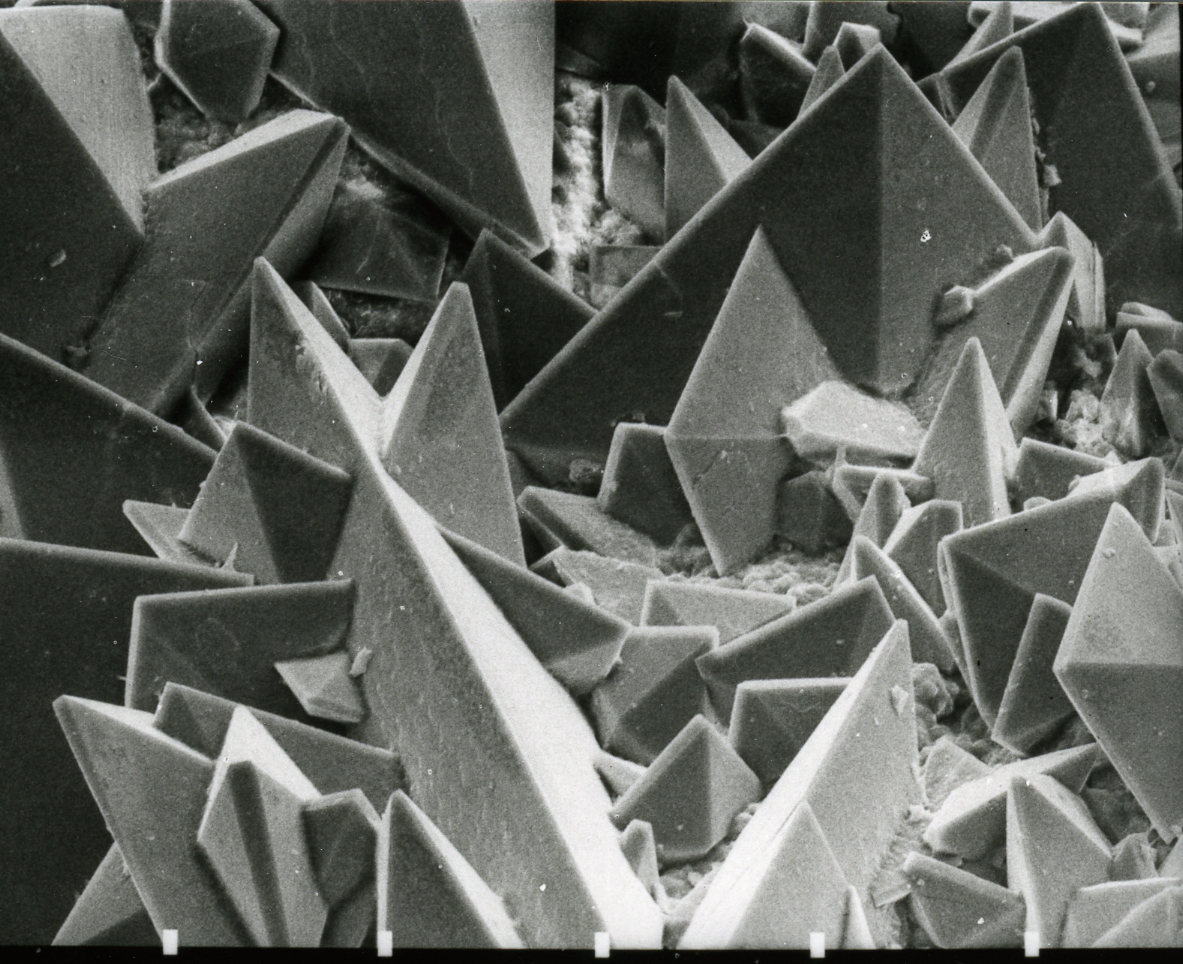
Samarium(III) Oxalate
Samarium(III) oxalate is an inorganic compound, a salt of samarium and oxalic acid with the formula Sm2(C2O4)3. The compound does not dissolve in water, forms a crystalline hydrate with yellow crystals. Synthesis Precipitation In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hai ... of soluble samarium salts with oxalic acid: ::\mathsf Also a reaction of samarium nitrate and oxalic acid in an aqueous solution: ::\mathsf Physical properties Samarium(III) oxalate forms a crystalline hydrate of the composition Sm2(C2O4)3 • 10H2O with yellow crystals. Chemical properties Decomposes on heating: ::\mathsf Crystalline hydrate Sm2(C2O4)3 • 10H2O decomposes stepwise. References {{Oxalates Oxalates Samarium(III) compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Praseodymium Oxalate
Praseodymium(III) oxalate is an inorganic compound, a salt of praseodymium metal and oxalic acid with the chemical formula C6O12Pr2. The compound forms light green crystals, insoluble in water, also forms crystalline hydrates. Synthesis The reaction of soluble praseodymium salts with oxalic acid Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...: ::\mathsf Properties Praseodymium oxalate forms light green crystals. It is poorly soluble in water. The compound forms crystalline hydrates (light green crystals): Pr2(C2O4)3•10H2O. The crystalline hydrate decomposes stepwise when heated: ::\mathsf Uses The compound is used as an intermediate product in the synthesis of praseodymium. It is also applied to colour some glasses and enamels. If mixed with certain other materials, the com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samarium(II) are also known, most notably the monoxide SmO, monochalcogenides SmS, SmSe and SmTe, as well as samarium(II) iodide. The last compound is a common reducing agent in chemical synthesis. Samarium has no significant biological role, and some samarium salts are slightly toxic. Samarium was discovered in 1879 by French chemist Paul-Émile Lecoq de Boisbaudran and named after the mineral samarskite from which it was isolated. The mineral itself was named after a Russian mine official, Colonel Vassili Samarsky-Bykhovets, who thus became the first person to have a chemical element named after him, albeit indirectly. Though classified as a rare-earth element, samarium is the 40th most abundant element in Earth's crust and more common th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus '' Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in facilities, acquisitio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (chemistry)
In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a super-saturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the ''precipitant''. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the 'supernate' or 'supernatant'. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (''e.g.'', metallurgy and alloys) when solid impurities segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical Chemistry (journal)
''Analytical Chemistry'' is a biweekly peer-reviewed scientific journal published since 1929 by the American Chemical Society. Articles address general principles of chemical measurement science and novel analytical methodologies. Topics commonly include chemical reactions and selectivity, chemometrics and data processing, electrochemistry, elemental and molecular characterization, imaging, instrumentation, mass spectrometry, microscale and nanoscale systems, -omics, sensing, separations, spectroscopy, and surface analysis. It is abstracted and indexed in Chemical Abstracts Service, CAB International, EBSCOhost, ProQuest, PubMed, Scopus, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', it has a 2020 impact factor of 6.986 . The editor-in-chief is Jonathan V. Sweedler (University of Illinois). See also *List of chemistry journals References External links * {{DEFAULTSORT:Analytical Chemistry (Journal) Analytical Chemistry Analytical c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalates
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate (C2O4(CH3)2). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a stepwise manner; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is (p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and only tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |