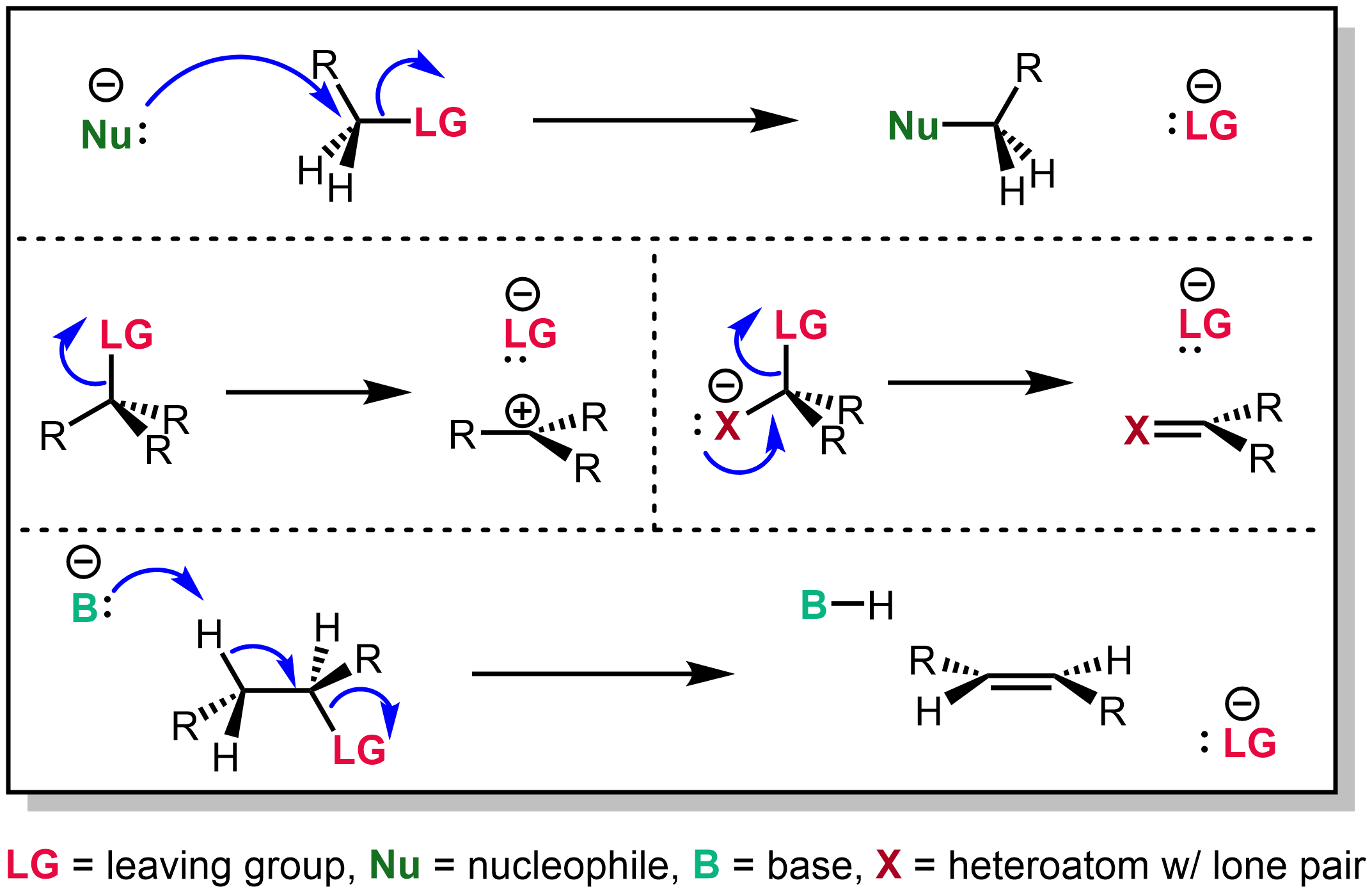
|
SN10
The unimolecular nucleophilic substitution (SN1) reaction is a substitution reaction in organic chemistry. The Hughes-Ingold symbol of the mechanism expresses two properties—"SN" stands for "nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with Alcohol (chemistry), secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction, SN2 reaction occurs. In inorganic chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NS1 Reaction Part2 Recombination Carbocation Nucleophile
NS1, NS 1, NS-1, NS.1, or ''variation'', may refer to: Non-structural protein 1 * NS1 influenza protein * NS1 dengue protein, used for NS1 antigen test * Human bocavirus NS1 * Carnivore bocaparvovirus 1 NS1 * Japanese encephalitis virus NS1 * Minute virus of mice NS1 * West Nile virus NS1 * Yellow fever virus NS1 Places * Jurong East MRT station (station code: NS1), Jurong East, Singapore * Kawanishi-Noseguchi Station (station code: NS01), Kawanishi, Hyōgo Prefecture, Japan * Ōmiya Station (Saitama) (station code: NS01), Ōmiya-ku, Saitama, Japan * Annapolis (provincial electoral district), constituency ''N.S. 01''; Nova Scotia, Canada Aerospace * U.S.S. NS-1, a U.S. Navy airship; see British blimps operated by the USN * RAF N.S. 1, a British NS class airship * Spartan NS-1, U.S. military trainer biplane * Stearman NS-1, U.S. military trainer biplane * New Shepard 1, a Blue Origin reusable space launch vehicle booster rocket (booster #1) * Blue Origin NS-1, a 2015 Ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for Chemical polarity#Polarity of molecules, polar molecules, and the most common solvent used by living things; all the ions and proteins in a Cell (biology), cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for Organic compound, organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents (D-limonene, citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Attack Of Oxonium Ion
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged Atomic nucleus, atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as Alcohol (chemistry), alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem , a place in Finland
{{disambig ...
Chem may refer to: *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" *Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel * CHEM-DT, a Canadian television channel See also * Chemo (other) * Kem (other) *Kemi Kemi (; ; ; ) is a cities of Finland, town and municipalities of Finland, municipality of Finland. It is located approximately from the city of Tornio and the Finland–Sweden border, Swedish border. The distance to Oulu is to the south and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm. Natural occurrence Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper. Formation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-butanol
''tert''-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as ''t''-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. ''tert''-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether. Natural occurrence ''tert''-Butyl alcohol has been identified in beer and chickpeas. It is also found in cassava, which is used as a fermentation ingredient in certain alcoholic beverages. Preparation ''tert''-Butyl alcohol is derived commercially from isobutane as a coproduct of propylene oxide production. It can also be produced by the catalytic hydration of isobutylene, or by a Grignard reaction between acetone Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |