|
SIBLING Proteins
The family of non-collagenous proteins known as SIBLING proteins, standing for small integrin-binding ligand, N-linked glycoprotein, are components of the extracellular matrix of bone and dentin. Evidence shows that these proteins play key roles in the mineralization of these tissues. The following are categorized as SIBLING proteins: # osteopontin (OPN) # bone sialoprotein Bone sialoprotein (BSP) is a component of mineralized tissues such as bone, dentin, cementum and calcified cartilage. BSP is a significant component of the bone extracellular matrix and has been suggested to constitute approximately 8% of all ... (BSP) # dentin matrix protein 1 (DMP1) # dentin sialophosphoprotein (DSPP) # matrix extracellular phosphoglycoprotein (MEPE) The genes coding for members of the SIBLING protein family are similarly organized and are all located on human chromosome 4q21-23. References {{Reflist Glycoproteins Extracellular matrix proteins Protein families ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of biomineralization, mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the Gut (anatomy), gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-linked Glycoprotein
''N''-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), in a process called ''N''-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of ''N''-linked glycans. Energetics of bond formation There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type of connective tissue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bone
A bone is a rigid organ that constitutes part of the skeleton in most vertebrate animals. Bones protect the various other organs of the body, produce red and white blood cells, store minerals, provide structure and support for the body, and enable mobility. Bones come in a variety of shapes and sizes and have complex internal and external structures. They are lightweight yet strong and hard and serve multiple functions. Bone tissue (osseous tissue), which is also called bone in the uncountable sense of that word, is hard tissue, a type of specialised connective tissue. It has a honeycomb-like matrix internally, which helps to give the bone rigidity. Bone tissue is made up of different types of bone cells. Osteoblasts and osteocytes are involved in the formation and mineralisation of bone; osteoclasts are involved in the resorption of bone tissue. Modified (flattened) osteoblasts become the lining cells that form a protective layer on the bone surface. The mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
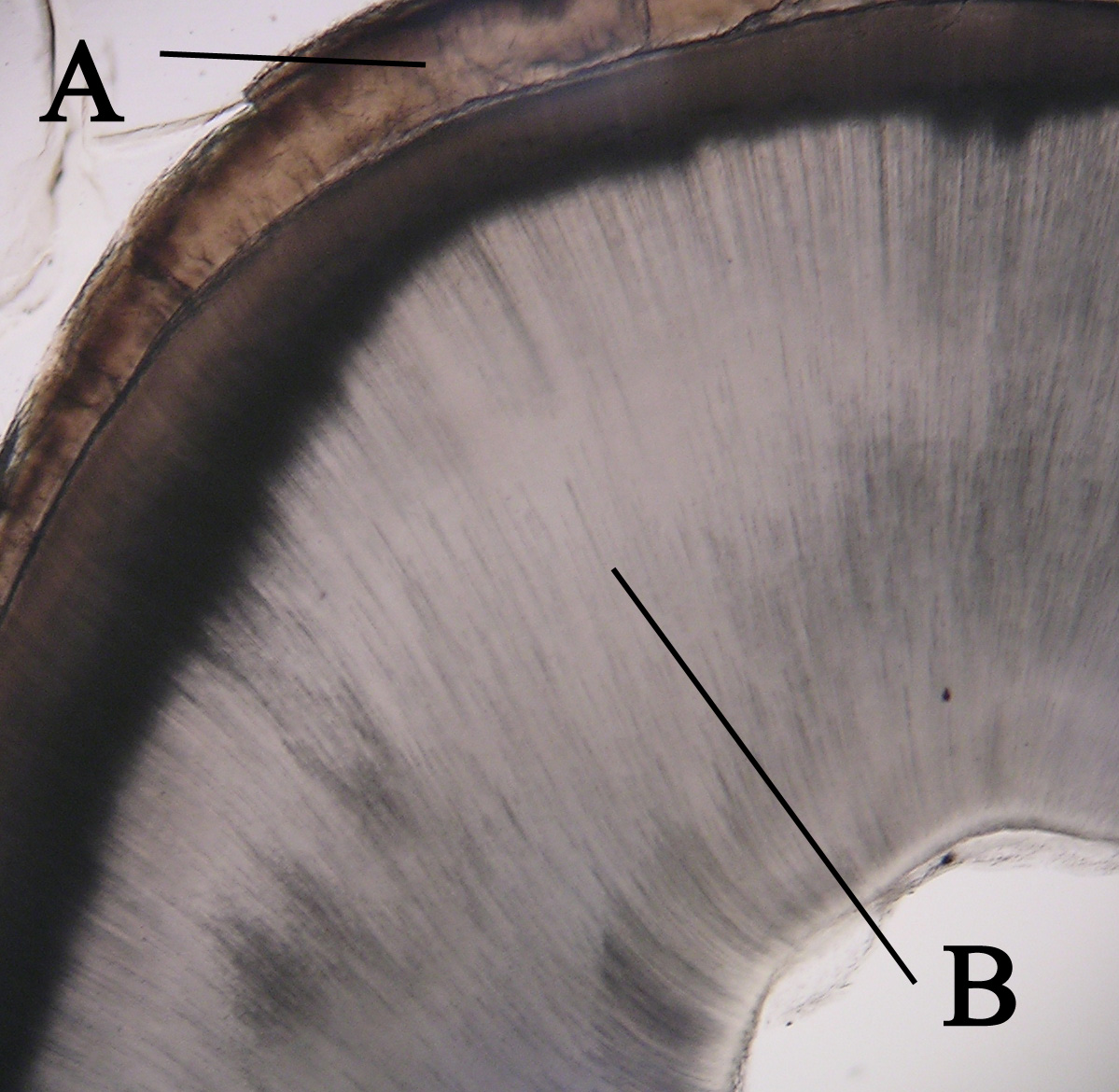
Dentin
Dentin ( ) (American English) or dentine ( or ) (British English) () is a calcified tissue (biology), tissue of the body and, along with tooth enamel, enamel, cementum, and pulp (tooth), pulp, is one of the four major components of teeth. It is usually covered by enamel on the crown and cementum on the root and surrounds the entire pulp. By volume, 45% of dentin consists of the mineral hydroxyapatite, 33% is organic material, and 22% is water. Yellow in appearance, it greatly affects the color of a tooth due to the translucency of enamel. Dentin, which is less mineralized and less brittle than enamel, is necessary for the support of enamel. Dentin rates approximately 3 on the Mohs scale of mineral hardness. There are two main characteristics which distinguish dentin from enamel: firstly, dentin forms throughout life; secondly, dentin is sensitive and can become hypersensitive to changes in temperature due to the sensory function of odontoblasts, especially when enamel recedes an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineralization (biology)
Biomineralization, also written biomineralisation, is the process by which living organisms produce minerals, often resulting in hardened or stiffened '' mineralized tissues''. It is an extremely widespread phenomenon: all six taxonomic kingdoms contain members that can form minerals, and over 60 different minerals have been identified in organisms. Examples include silicates in algae and diatoms, carbonates in invertebrates, and calcium phosphates and carbonates in vertebrates. These minerals often form structural features such as sea shells and the bone in mammals and birds. Organisms have been producing mineralized skeletons for the past 550 million years. Calcium carbonates and calcium phosphates are usually crystalline, but silica organisms (such as sponges and diatoms) are always non-crystalline minerals. Other examples include copper, iron, and gold deposits involving bacteria. Biologically formed minerals often have special uses such as magnetic sensors in magnetota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osteopontin
Osteopontin (OPN), also known as bone /sialoprotein I (BSP-1 or BNSP), early T-lymphocyte activation (ETA-1), secreted phosphoprotein 1 (SPP1), 2ar and Rickettsia resistance (Ric), is a protein that in humans is encoded by the ''SPP1'' gene (secreted phosphoprotein 1). The murine ortholog is ''Spp1''. Osteopontin is a SIBLING (glycoprotein) that was first identified in 1986 in osteoblasts. The prefix '' osteo-'' indicates that the protein is expressed in bone, although it is also expressed in other tissues. The suffix ''-pontin'' is derived from “pons,” the Latin word for bridge, and signifies osteopontin's role as a linking protein. Osteopontin is an extracellular structural protein and therefore an organic component of bone. The gene has 7 exons, spans 5 kilobases in length and in humans it is located on the long arm of chromosome 4 region 22 (4q1322.1). The protein is composed of ~300 amino acids residues and has ~30 carbohydrate residues attached, including 10 sia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bone Sialoprotein
Bone sialoprotein (BSP) is a component of mineralized tissues such as bone, dentin, cementum and calcified cartilage. BSP is a significant component of the bone extracellular matrix and has been suggested to constitute approximately 8% of all non-collagenous proteins found in bone and cementum. BSP, a SIBLING protein, was originally isolated from bovine cortical bone as a 23-kDa glycopeptide with high sialic acid content. The human variant of BSP is called bone sialoprotein 2 also known as cell-binding sialoprotein or integrin-binding sialoprotein and is encoded by the ''IBSP'' gene. Structure Native BSP has an apparent molecular weight of 60-80 kDa based on SDS-PAGE, which is a considerable deviation from the predicted weight (based on cDNA sequence) of approximately 33 kDa. The mammalian BSP cDNAs encode for proteins averaging 317 amino acids, which includes the 16-residue preprotein secretory signal peptide. Among the mammalian cDNAs currently characterized, there is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DMP1 (gene)
Dentin matrix acidic phosphoprotein 1 is a protein that in humans is encoded by the ''DMP1'' gene. Dentin matrix acidic phosphoprotein is an extracellular matrix protein and a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family (other members being DSPP, IBSP, MEPE, and SPP1). This protein, which is critical for proper mineralization of bone and dentin, is present in diverse cells of bone and tooth tissues. The protein contains a large number of acidic domains, multiple phosphorylation sites, a functional Arg-Gly-Asp cell attachment sequence, and a DNA binding domain. In undifferentiated osteoblasts it is primarily a nuclear protein that regulates the expression of osteoblast-specific genes. During osteoblast maturation the protein becomes phosphorylated and is exported to the extracellular matrix, where it orchestrates mineralized matrix formation. Mutations in the gene are known to cause autosomal recessive hypophosphatemia, a disease that mani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dentin Sialophosphoprotein (protein)
Dentin sialophosphoprotein is a precursor protein for other proteins found in the teeth. It is produced by cells (odontoblasts) inside the teeth (dental pulp), and in smaller quantities by bone tissues (osteoblasts and osteocytes). It is required for normal hardening (mineralisation) of teeth. During teeth development, it is broken down into three proteins such as dentin sialoprotein (DSP), dentin glycoprotein (DGP), and dentin phosphoprotein (DPP). These proteins become the major non-collagenous components of teeth. Their distribution in the collagen matrix of the forming dentin suggests these proteins play an important role in the regulation of mineral deposition. Additional evidence for this correlation is phenotypically manifested in patients with mutant forms of dentin sialophosphoprotein. Such patients suffer dental anomalies including type III dentinogenesis imperfecta Dentinogenesis imperfecta (DI) is a genetic disorder of Human tooth development, tooth development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEPE
''Mepe'' (Old Georgian: ႫႴ; ka, მეფე ; ) is a royal title used to designate the Georgian monarch, whether it is referring to a king or a queen regnant. The title was originally a male ruling title. Etymology The word is derived from Georgian word მეუფე (''meupe'') which literally means sovereign and lord. Some Georgian dialects has the term as ნეფე (''nepe''), all derived from common Proto-Kartvelian მფ/მეფე/მაფა (''mp/mepe/mapa''). Even though ''mepe'' has a female equivalent, დედოფალი (''dedopali;'' ) it is only applied to the king's consort and does not have a meaning of a ruling monarch. History The term ''mepe'' was utilized since pre-Christian beginnings with Azo, but the role would get more structured during the reign of Pharnavaz I in the 3rd century BC. His successors, the Pharnavazid ''mepes'' would be titled as ''goliath'' who would possess 𐬓𐬀𐬭𐬆𐬥𐬀𐬵 ('' pharnah''; ), the divinely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |