|
Rydberg Correction
The term quantum defect refers to two concepts: energy loss in lasers and energy levels in alkali elements. Both deal with quantum systems where matter interacts with light. In laser science In laser science, the term quantum defect refers to the fact that the energy of a pump photon is generally higher than that of a ''signal photon'' (photon of the output radiation). The energy difference is lost to heat, which may carry away the excess entropy delivered by the multimode incoherent pump. The quantum defect of a laser can be defined as the part of the energy of the pumping photon which is lost (not turned into photons at the lasing wavelength) in the gain medium during lasing. At given frequency \omega_ of pump and given frequency \omega_ of lasing, the quantum defect q = \hbar \omega_ - \hbar\omega_. Such a quantum defect has dimensions of energy; for the efficient operation, the temperature of the gain medium (measured in units of energy) should be small compared to the qua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
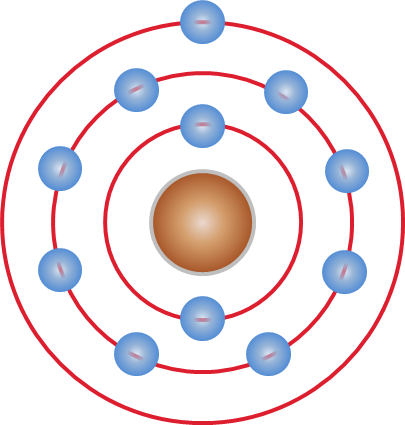
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised Homologous series, homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, hardness, sof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Efficiency Of A Solar Cell
Solar-cell efficiency is the portion of energy in the form of sunlight that can be converted via photovoltaics into electricity by the solar cell. The efficiency of the solar cells used in a photovoltaic system, in combination with latitude and climate, determines the annual energy output of the system. For example, a solar panel with 20% efficiency and an area of 1 m2 produces 200 kWh/yr at Standard Test Conditions if exposed to the Standard Test Condition solar irradiance value of 1000 W/m2 for 2.74 hours a day. Usually solar panels are exposed to sunlight for longer than this in a given day, but the solar irradiance is less than 1000 W/m2 for most of the day. A solar panel can produce more when the Sun is high in Earth's sky and produces less in cloudy conditions, or when the Sun is low in the sky. The Sun is lower in the sky in the winter. Two location dependent factors that affect solar PV yield are the dispersion and intensity of solar radiation. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
External Quantum Efficiency
The term quantum efficiency (QE) may apply to incident photon to converted electron (IPCE) ratio of a photosensitive device, or it may refer to the TMR effect of a magnetic tunnel junction. This article deals with the term as a measurement of a device's electrical sensitivity to light. In a charge-coupled device (CCD) or other photodetector, it is the ratio between the number of charge carriers collected at either terminal and the number of photons hitting the device's photoreactive surface. As a ratio, QE is dimensionless, but it is closely related to the responsivity, which is expressed in amps per watt. Since the energy of a photon is inversely proportional to its wavelength, QE is often measured over a range of different wavelengths to characterize a device's efficiency at each photon energy level. For typical semiconductor photodetectors, QE drops to zero for photons whose energy is below the band gap. A photographic film typically has a QE of much less than 10%, while CCD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised Homologous series, homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all shiny, hardness, sof ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Formula
In atomic physics, the Rydberg formula calculates the wavelengths of a spectral line in many chemical elements. The formula was primarily presented as a generalization of the Balmer series for all atomic electron transitions of hydrogen. It was first empirically stated in 1888 by the Swedish physicist Johannes Rydberg,See: * * English summary: then theoretically by Niels Bohr in 1913, who used a primitive form of quantum mechanics. The formula directly generalizes the equations used to calculate the wavelengths of the hydrogen spectral series. History In 1890, Rydberg proposed on a formula describing the relation between the wavelengths in spectral lines of alkali metals. He noticed that lines came in series and he found that he could simplify his calculations using the wavenumber (the number of waves occupying the unit length, equal to 1/''λ'', the inverse of the wavelength) as his unit of measurement. He plotted the wavenumbers (''n'') of successive lines in each series a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wavefunction
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter), psi, respectively). Wave functions are complex number, complex-valued. For example, a wave function might assign a complex number to each point in a region of space. The Born rule provides the means to turn these complex probability amplitudes into actual probabilities. In one common form, it says that the squared modulus of a wave function that depends upon position is the probability density function, probability density of measurement in quantum mechanics, measuring a particle as being at a given place. The integral of a wavefunction's squared modulus over all the system's degrees of freedom must be equal to 1, a condition called ''normalization''. Since the wave function is complex-valued, only its relative phase and relative magnitud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angular Momentum Operator
In quantum mechanics, the angular momentum operator is one of several related operators analogous to classical angular momentum. The angular momentum operator plays a central role in the theory of atomic and molecular physics and other quantum problems involving rotational symmetry. Being an observable, its eigenfunctions represent the distinguishable physical states of a system's angular momentum, and the corresponding eigenvalues the observable experimental values. When applied to a mathematical representation of the state of a system, yields the same state multiplied by its angular momentum value if the state is an eigenstate (as per the eigenstates/eigenvalues equation). In both classical and quantum mechanical systems, angular momentum (together with linear momentum and energy) is one of the three fundamental properties of motion.Introductory Quantum Mechanics, Richard L. Liboff, 2nd Edition, There are several angular momentum operators: total angular momentum (usually den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Principal Quantum Number
In quantum mechanics, the principal quantum number (''n'') of an electron in an atom indicates which electron shell or energy level it is in. Its values are natural numbers (1, 2, 3, ...). Hydrogen and Helium, at their lowest energies, have just one electron shell. Lithium through Neon (see periodic table) have two shells: two electrons in the first shell, and up to 8 in the second shell. Larger atoms have more shells. The principal quantum number is one of four quantum numbers assigned to each electron in an atom to describe the quantum state of the electron. The other quantum numbers for bound electrons are the total angular momentum of the orbit ''ℓ'', the angular momentum in the z direction ''ℓz'', and the spin of the electron ''s''. Overview and history As ''n'' increases, the electron is also at a higher energy and is, therefore, less tightly bound to the nucleus. For higher ''n'', the electron is farther from the nucleus, on average. For each value of ''n'', th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Speed Of Light
The speed of light in vacuum, commonly denoted , is a universal physical constant exactly equal to ). It is exact because, by international agreement, a metre is defined as the length of the path travelled by light in vacuum during a time interval of second. The speed of light is invariant (physics), the same for all observers, no matter their relative velocity. It is the upper limit for the speed at which Information#Physics_and_determinacy, information, matter, or energy can travel through Space#Relativity, space. All forms of electromagnetic radiation, including visible light, travel at the speed of light. For many practical purposes, light and other electromagnetic waves will appear to propagate instantaneously, but for long distances and sensitive measurements, their finite speed has noticeable effects. Much starlight viewed on Earth is from the distant past, allowing humans to study the history of the universe by viewing distant objects. When Data communication, comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Planck Constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a matter wave equals the Planck constant divided by the associated particle momentum. The constant was postulated by Max Planck in 1900 as a proportionality constant needed to explain experimental black-body radiation. Planck later referred to the constant as the "quantum of Action (physics), action". In 1905, Albert Einstein associated the "quantum" or minimal element of the energy to the electromagnetic wave itself. Max Planck received the 1918 Nobel Prize in Physics "in recognition of the services he rendered to the advancement of Physics by his discovery of energy quanta". In metrology, the Planck constant is used, together with other constants, to define the kilogram, the SI unit of mass. The SI units are defined in such a way that, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rydberg Constant
In spectroscopy, the Rydberg constant, symbol R_\infty for heavy atoms or R_\text for hydrogen, named after the Swedish physicist Johannes Rydberg, is a physical constant relating to the electromagnetic spectra of an atom. The constant first arose as an empirical fitting parameter in the Rydberg formula for the hydrogen spectral series, but Niels Bohr later showed that its value could be calculated from more fundamental constants according to his model of the atom. Before the 2019 revision of the SI, R_\infty and the electron spin ''g''-factor were the most accurately measured physical constants. The constant is expressed for either hydrogen as R_\text, or at the limit of infinite nuclear mass as R_\infty. In either case, the constant is used to express the limiting value of the highest wavenumber (inverse wavelength) of any photon that can be emitted from a hydrogen atom, or, alternatively, the wavenumber of the lowest-energy photon capable of ionizing a hydrogen at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |