|
RU-16117
RU-16117 is an estrogen medication which was investigated for the potential treatment of symptoms of estrogen deficiency such as hot flashes and osteoporosis in women but was never marketed. It was developed for use by mouth. Pharmacology Pharmacodynamics RU-16117 is an estrogen, or an agonist of the estrogen receptor (ER). In mouse uterine tissue, it shows about 5 to 13% of the affinity of estradiol for the ER and about 1% of the estrogenic activity of estradiol. Conversely, it shows no affinity for the androgen, progesterone, glucocorticoid, and mineralocorticoid receptors, nor any activities associated with interactions with these receptors. While the association rate of RU-16117 to the ER is the same as that of moxestrol, it dissociates from the ER extremely rapidly at rates of about three times faster than estradiol and about 20 times faster than moxestrol. This is similar to the case of estriol, which RU-16117 is described as sharing similarities with. RU-16117 is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moxestrol
Moxestrol, sold under the brand name Surestryl, is an estrogen medication which has been used in Europe for the treatment of menopausal symptoms and menstrual disorders. It is taken by mouth. In addition to its use as a medication, moxestrol has been used in scientific research as a radioligand of the estrogen receptor. Medical uses Moxestrol is or has been used in the treatment of menopausal symptoms and menstrual disorders. It has been used at dosages of 50 to 150 µg per week for long-term therapy to 25 to 250 µg per day for short-term therapy. Pharmacology Pharmacodynamics Moxestrol is an estrogen, or an agonist of the estrogen receptors. It is the 11β-methoxy derivative of ethinylestradiol and is one of the most potent estrogens known, being some 10 to 100 times more potent than estradiol and about 5-fold more potent than ethinylestradiol. The very high potency of moxestrol has been attributed to its high affinity for the estrogen receptor (ER), its negligible plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progesterone Receptor
The progesterone receptor (PR), also known as NR3C3 or nuclear receptor subfamily 3, group C, member 3, is a protein found inside cells. It is activated by the steroid hormone progesterone. In humans, PR is encoded by a single ''PGR'' gene residing on chromosome 11q22, it has two isoforms, PR-A and PR-B, that differ in their molecular weight. The PR-B is the positive regulator of the effects of progesterone, while PR-A serve to antagonize the effects of PR-B. Mechanism Progesterone is necessary to induce the progesterone receptors. When no binding hormone is present the carboxyl terminal inhibits transcription. Binding to a hormone induces a structural change that removes the inhibitory action. Progesterone antagonists prevent the structural reconfiguration. After progesterone binds to the receptor, restructuring with dimerization follows and the complex enters the nucleus and binds to DNA. There transcription takes place, resulting in formation of messenger RNA that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Progesterone (medication)
Progesterone (P4) is a medication and naturally occurring steroid hormone. It is a progestogen and is used in combination with estrogens mainly in hormone therapy for menopausal symptoms and low sex hormone levels in women. It is also used in women to support pregnancy and fertility and to treat gynecological disorders. Progesterone can be taken by mouth, in through the vagina, and by injection into muscle or fat, among other routes. A progesterone vaginal ring and progesterone intrauterine device used for birth control also exist in some areas of the world. Progesterone is well tolerated and often produces few or no side effects. However, a number of side effects are possible, for instance mood changes. If progesterone is taken by mouth or at high doses, certain central side effects including sedation, sleepiness, and cognitive impairment can also occur. The medication is a naturally occurring progestogen and hence is an agonist of the progesterone receptor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethinylestradiol
Ethinylestradiol (EE) is an estrogen medication which is used widely in birth control pills in combination with progestins. In the past, EE was widely used for various indications such as the treatment of menopausal symptoms, gynecological disorders, and certain hormone-sensitive cancers. It is usually taken by mouth but is also used as a patch and vaginal ring. The general side effects of EE include breast tenderness and enlargement, headache, fluid retention, and nausea among others. In men, EE can additionally cause breast development, feminization in general, hypogonadism, and sexual dysfunction. Rare but serious side effects include blood clots, liver damage, and cancer of the uterus. EE is an estrogen, or an agonist of the estrogen receptors, the biological target of estrogens like estradiol. It is a synthetic derivative of estradiol, a natural estrogen, and differs from it in various ways. Compared to estradiol, EE has greatly improved bioavaila ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estriol (medication)
Estriol (E3), sold under the brand name Ovestin among others, is an estrogen medication and naturally occurring steroid hormone which is used in menopausal hormone therapy. It is also used in veterinary medicine as Incurin to treat urinary incontinence due to estrogen deficiency in dogs. The medication is taken by mouth in the form of tablets, as a cream that is applied to the skin, as a cream or pessary that is applied in the vagina, and by injection into muscle. Estriol is well- tolerated and produces relatively few adverse effects. Side effects may include breast tenderness, vaginal discomfort and discharge, and endometrial hyperplasia. Estriol is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol. It is an atypical and relatively weak estrogen, with much lower potency than estradiol. When present continuously at adequate concentrations however, estriol produces full est ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
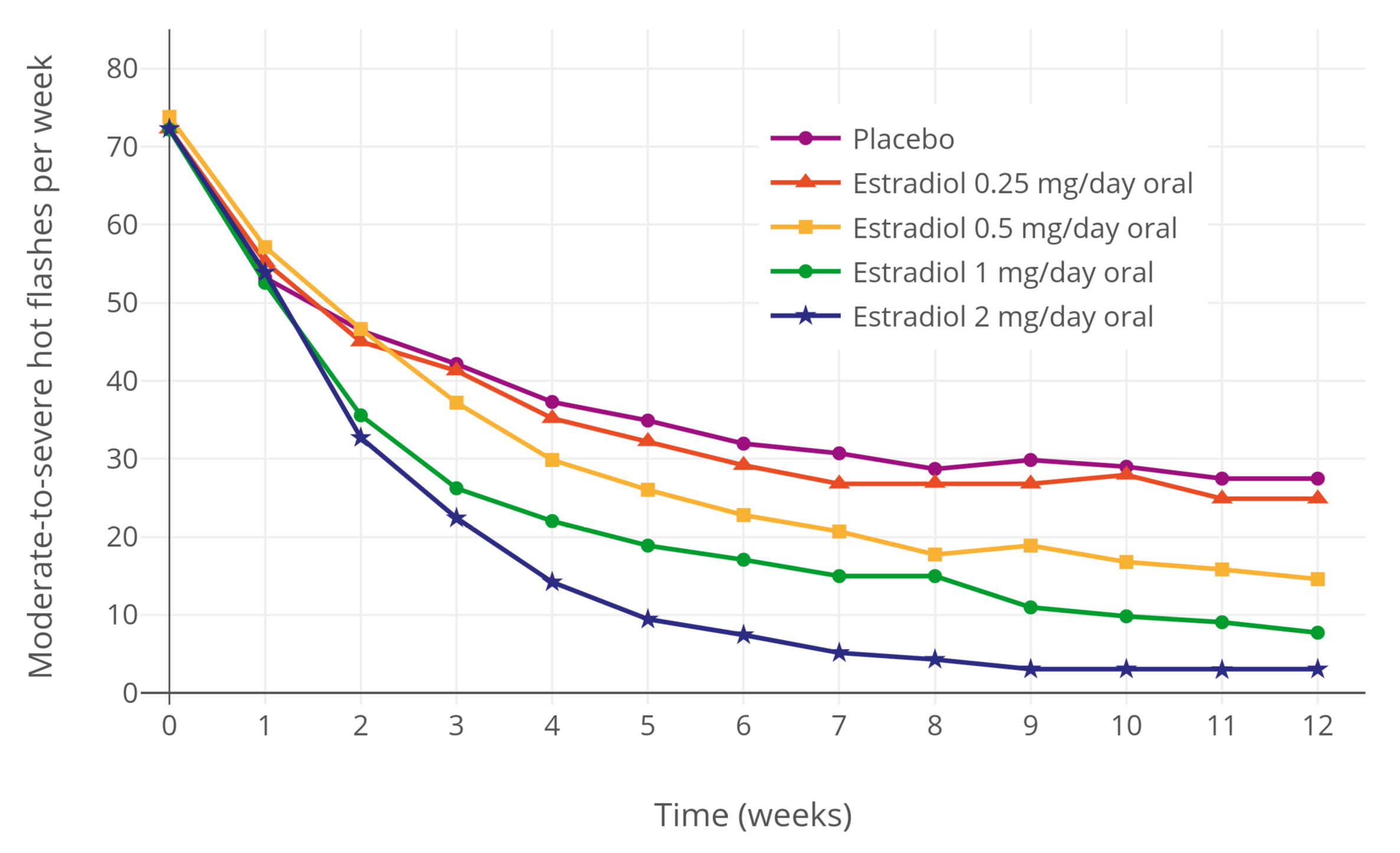
Estradiol (medication)
Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes. Side effects of estradiol in women include breast tenderness, breast enlargement, headache, fluid retention, and nausea among others. Men and children who are exposed to estradiol may develop symptoms of feminization, such as breast development and a feminine pattern of fat distribution, and men may also experience ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uterotrophic
The uterus (from Latin ''uterus'', plural ''uteri'') or womb () is the organ in the reproductive system of most female mammals, including humans that accommodates the embryonic and fetal development of one or more embryos until birth. The uterus is a hormone-responsive sex organ that contains glands in its lining that secrete uterine milk for embryonic nourishment. In the human, the lower end of the uterus, is a narrow part known as the isthmus that connects to the cervix, leading to the vagina. The upper end, the body of the uterus, is connected to the fallopian tubes, at the uterine horns, and the rounded part above the openings to the fallopian tubes is the fundus. The connection of the uterine cavity with a fallopian tube is called the uterotubal junction. The fertilized egg is carried to the uterus along the fallopian tube. It will have divided on its journey to form a blastocyst that will implant itself into the lining of the uterus – the endometrium, where it will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiestrogen
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production., Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development. Antiandrogens are sex hormone antagonists which are able to lower the production and the effects that testosterone can have on female bodies. Types and examples Antiestrogens include selective estrogen receptor modulators (SERMs) like tamoxifen, clomifene, and raloxifene, the ER silent antagonist and selective estrogen receptor degrader (SERD) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Agonist
In pharmacology, partial agonists are drugs that bind to and activate a given receptor, but have only partial efficacy at the receptor relative to a full agonist. They may also be considered ligands which display both agonistic and antagonistic effects—when both a full agonist and partial agonist are present, the partial agonist actually acts as a competitive antagonist , competing with the full agonist for receptor occupancy and producing a net decrease in the receptor activation observed with the full agonist alone. Clinically, partial agonists can be used to activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present. Some currently common drugs that have been classed as partial agonists at particular receptors include buspirone, aripiprazole, buprenorphine, nalmefene and norclozapine. Examples of ligan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |