|
QS-21
QS-21 is a purified plant extract used as a vaccine adjuvant. It is derived from the soap bark tree (''Quillaja saponaria''), which is native to the countries of Chile, Peru, and Bolivia. The crude drug (''Quillajae cortex'', Quillaia) is imported from Peru and Chile. The extract contains water-soluble triterpene glycosides, which are members of a family of plant-based compounds called saponins. It has been tested as an adjuvant in various vaccines in attempts to improve their efficacy. It is believed to enhance both humoral and cell-mediated immunity. Use QS-21 has been clinically evaluated as a vaccine adjuvant. , it had been tested in more than 3000 patients in 60 clinical trials. Direct, unencapsuled QS-21 is very liable to hydrolysis. It also causes immediate pain at injection site and ''in vitro'' causes hemolysis. All of these can be prevented by packaging QS-21 into lipid-based particles, which also have the added advantage of targeting its delivery to phagocytes. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vaccine Adjuvant
In immunology, an adjuvant is a substance that increases or modulates the immune response to a vaccine. The word "adjuvant" comes from the Latin word , meaning to help or aid. "An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens." In the early days of vaccine manufacture, significant variations in the efficacy of different batches of the same vaccine were correctly assumed to be caused by contamination of the reaction vessels. However, it was soon found that more scrupulous cleaning actually seemed to ''reduce'' the effectiveness of the vaccines, and some contaminants actually enhanced the immune response. There are many known adjuvants in widespread use, including potassium alum, various plant and animal derived oils and virosomes. Overview Adjuvants in immunology are often used to modify or augment the effects of a vaccine by stimulating the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix-M
Matrix-M is a vaccine adjuvant, a substance that is added to various vaccines to stimulate the immune response. It was patented in 2020 by Novavax and is composed of nanoparticles from saponins extracted from '' Quillaja saponaria'' (soapbark) trees, cholesterol, and phospholipids. It is an immune stimulating complex ( ISCOM), which are nanospheres formed when saponin is mixed with two types of fats. Composition Matrix-M contains a complex mix of saponins extracted from the bark of soapbark trees (Quillaia) packaged into nanoparticles made of cholesterol and phospholipids. 15% of the nanoparticles are known as Matrix-C and contain saponins derived from "Fraction C" of the tree bark extract (mainly QS-21). Matrix-C has strong adjuvant activity but is also highly reactogenic (lethargy and lethality in mice). The remaining 85% are known as Matrix-A and contain "Fraction A" saponins. Matrix-A is a weaker adjuvant but is also very well tolerated. Combined, they form a strong adjuvan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
M72/AS01E
M72/AS01E is an experimental tuberculosis vaccine. If approved, it would be the first vaccine for tuberculosis in more than a century after the BCG vaccine. The vaccine consists of two main ingredients. The antigen part is M72, a recombinant fusion protein derived from the sequences of two ''M. tuberculosis'' antigens (Mtb32A and Mtb39A ). The adjuvant part is AS01E, a combination of QS-21, cholesterol, and monophosphoryl lipid A (MPL). It was initially developed by GSK plc and funded by non-profits and governments due to the limited commercial potential of tuberculosis vaccines, which are mainly needed in poor countries. The vaccine candidate completed a Phase II clinical trial in 2018, but its development was stalled thereafter due to negotiations between GSK, which was uninterested in the late stage development of the vaccine candidate, and non-commercial backers. After almost two years, the company concluded an agreement with the Bill & Melinda Gates Medical Research Insti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzymatic, enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a ''C-glycoside'') glycosidic bond. Accord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RTS,S
RTS,S/AS01 (trade name Mosquirix) is a recombinant protein-based malaria vaccine. It is one of two malaria vaccines approved (the other is R21/Matrix-M). , the vaccine has been given to 1million children living in areas with moderate-to-high malaria transmission, with millions more doses to be provided as the vaccine's production expands. 18 million doses have been allocated for 2023-2025. It requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1–2 years. The vaccine reduces hospital admissions from severe malaria by around 30% and reduces toddler deaths by 15%. Medical uses RTS,S/AS01 (commercial name ''Mosquirix'') is the only malaria vaccine approved and in current use. The vaccine's use requires at least three doses in infants by age 2, with a fourth dose extending the protection for another 1–2 years. The vaccine reduces hospital admissions from severe malaria by around 30%. History Potential malaria vaccine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
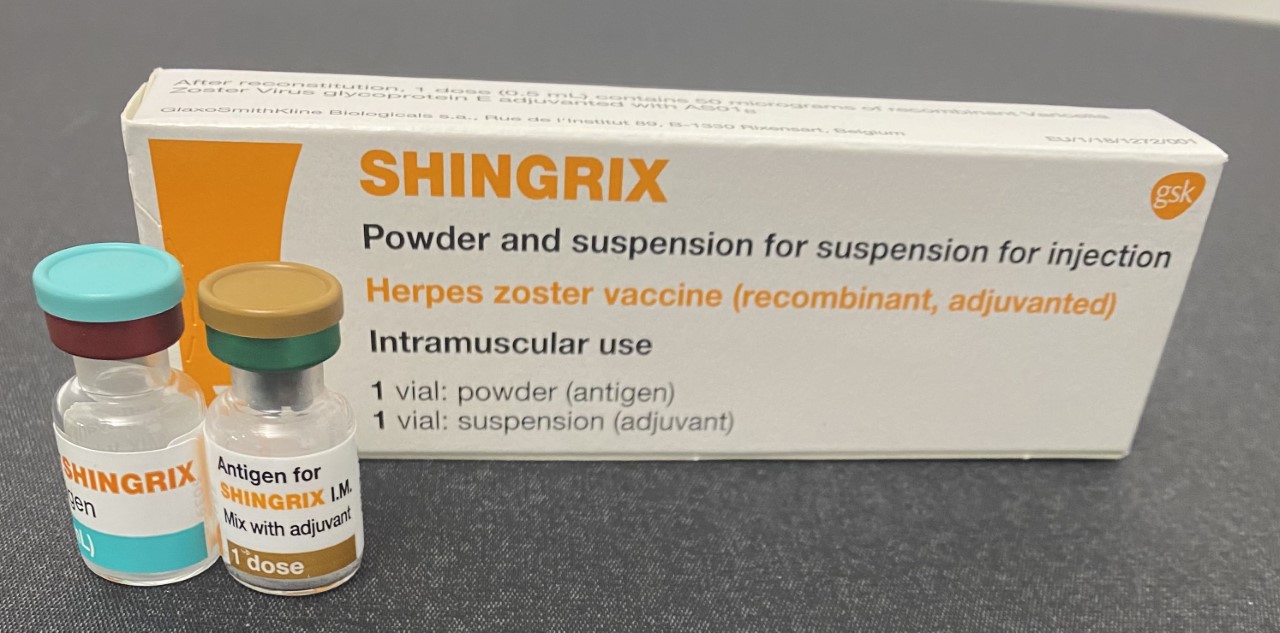
Shingrix
A zoster vaccine is a vaccine that reduces the incidence of herpes zoster (shingles), a disease caused by reactivation of the varicella zoster virus, which is also responsible for chickenpox. Shingles provokes a painful rash with blisters, and can be followed by chronic pain (postherpetic neuralgia), as well as other complications. Older people are more often affected, as are people with weakened immune systems (immunosuppression). Both shingles and postherpetic neuralgia can be prevented by vaccination. Two zoster vaccines have been approved for use in people over 50 years old. Shingrix ( GSK) is a recombinant subunit vaccine which has been used in many countries since 2017. Zostavax ( Merck), in use since 2006, is an attenuated vaccine which consists of a larger-than-normal dose of chickenpox vaccine. Unlike Shingrix, Zostavax is not suitable for people with immunosuppression or diseases that affect the immune system. Zostavax was discontinued in the United States in No ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phagocyte
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek ', "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek ''kutos,'' "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae.Ilya Mechnikov retrieved on November 28, 2008. Fro ''Physiology or Medicine 1901–192 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semi-synthesis
Semisynthesis, or partial chemical synthesis, is a type of chemical synthesis that uses chemical compounds isolated from natural sources (such as microbial cell cultures or plant material) as the starting materials to produce novel compounds with distinct chemical and medicinal properties. The novel compounds generally have a high molecular weight or a complex molecular structure, more so than those produced by total synthesis from simple starting materials. Semisynthesis is a means of preparing many medicines more cheaply than by total synthesis since fewer chemical steps are necessary. Drugs derived from natural sources are commonly produced either by isolation from their natural source or, as described here, through semisynthesis of an isolated agent. From the perspective of chemical synthesis, living organisms act as highly efficient chemical factories, capable of producing structurally complex compounds through biosynthesis. In contrast, engineered chemical synthesis, al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Defense Production Act Of 1950
The Defense Production Act (DPA) of 1950 () is a United States federal law enacted on September 8, 1950, in response to the start of the Korean War.Congressional Research ServiceThe Defense Production Act of 1950: History, Authorities, and Considerations for Congress, updated November 20, 2018, accessed January 17, 2019 fas.org It was part of a broad civil defense and war mobilization effort in the context of the Cold War. Its implementing regulations, the Defense Priorities and Allocation System (DPAS), are located at 15 CFR §§700 to 700.93. Since 1950, the act has been reauthorized over 50 times. It has been periodically amended and remains in force. Amendments The Defense Production Act (DPA) has undergone several amendments since it was first passed in 1950. These amendments have entailed alterations to its definition of "national defense." Four of the major amendments are described below: # In 1970, the existing definition of "national defense" was amended to inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CSL Limited
CSL Limited is an Australian multinational specialty biotechnology company that researches, develops, manufactures, and markets products to treat and prevent serious human medical conditions. CSL's product areas include blood plasma derivatives, vaccines, antivenom, and cell culture reagents used in various medical and genetic research and manufacturing applications. The company was established in 1916 as Commonwealth Serum Laboratories and was wholly owned by the Australian federal government until its privatisation in 1994. History Origin and Penfold directorship CSL was founded in 1916 as Commonwealth Serum Laboratories, an Australian government body focused on vaccine manufacture. Under the first director, William Penfold, CSL commenced operation in the vacant Walter and Eliza Hall Institute building at the Royal Melbourne Hospital in 1918 before moving to its purpose-built Parkville premises in the following year. Morgan directorship After ongoing disputes with th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |