|
Pyrrolizidine Alkaloidosis
Pyrrolizidine alkaloidosis is a disease caused by chronic poisoning found in humans and other animals caused by ingesting poisonous plants which contain the natural chemical compounds known as pyrrolizidine alkaloids.''The Merck Veterinary Manual''. Pyrrolizidine Alkaloidosis: Introduction (Seneciosis, Senecio poisoning, Ragwort toxicity). Merck & Co., Inc., 2008. Web. 15 November Pyrrolizidine alkaloidosis can result in damage to the liver, kidneys, heart, brain, smooth muscles, lungs, DNA, lesions all over the body, and could be a potential cause of cancer.Rizk, Abdel-Fattah M., ''Naturally Occurring Pyrrolizidine Alkaloids''. Doah: CRC Press, 1990 Pyrrolizidine alkaloidosis is known by many other names such as "Pictou Disease" in Canada and "Winton Disease" in New Zealand.Hirono, I. ''Naturally Occurring Carcinogens of Plant Origin''. Toyoake: Elsevier, 1987 Cereal crops and forage crops can sometimes become polluted with pyrrolizidine-containing seeds, resulting in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrolizidine Alkaloid
Pyrrolizidine alkaloids (PAs), sometimes referred to as necine bases, are a group of naturally occurring alkaloids based on the structure of pyrrolizidine. Pyrrolizidine alkaloids are produced by plants as a defense mechanism against insect herbivores. More than 660 PAs and PA N-oxides have been identified in over 6,000 plants, and about half of them exhibit hepatotoxicity. They are found frequently in plants in the Boraginaceae, Asteraceae, Orchidaceae and Fabaceae families; less frequently in the Convolvulaceae and Poaceae, and in at least one species in the Lamiaceae. It has been estimated that 3% of the world’s flowering plants contain pyrrolizidine alkaloids. Honey can contain pyrrolizidine alkaloids, as can grains, milk, offal and eggs. To date (2011), there is no international regulation of PAs in food, unlike those for herbs and medicines. Unsaturated pyrrolizidine alkaloids are hepatotoxic, that is, damaging to the liver. PAs also cause hepatic veno-occlusive d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
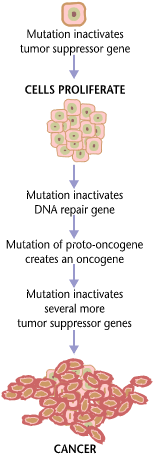
Carcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to canc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genotoxicity
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis. To assay for genotoxic molecules, researchers assay for DNA damage in cells exposed to the toxic substrates. This DNA damage can be in the form of single- and double-strand breaks, loss of excision repair, cross-linking, alkali-labile sites, point mutations, and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adduct
An adduct (from the Latin ''adductus'', "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can just be considered as a single product resulting from the direct combination of different molecules which comprises all the reactant molecules' atoms. Adducts often form between Lewis acids and Lewis bases. A good example is the formation of adducts between the Lewis acid borane and the oxygen atom in the Lewis bases, tetrahydrofuran (THF): BH3·O(CH2)4 or diethyl ether: BH3·O(CH3CH2)2. Many Lewis acids and Lewis bases reacting in the gas phase or in non-aqueous solvents to form adducts have been examined in the ECW model. Trimethylboron, trimethyltin chloride and bis(hex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver Proteins
The liver plays the major role in producing proteins that are secreted into the blood, including major plasma proteins, factors in hemostasis and fibrinolysis, carrier proteins, hormones, prohormones and apolipoprotein: Major plasma proteins All plasma proteins except Gamma-globulins are synthesised in the liver. *Human serum albumin, osmolyte and carrier protein *α-fetoprotein, the fetal counterpart of serum albumin *Soluble plasma fibronectin, forming a blood clot that stops bleeding *C-reactive protein, opsonin on microbes,Lippincott's Illustrated Reviews: Immunology. Paperback: 384 pages. Publisher: Lippincott Williams & Wilkins; (July 1, 2007). Language: English. . Page 182 acute phase protein *Various other globulins Factors in hemostasis and fibrinolysis *Stimulators of coagulation: **All factors in the coagulation cascade. **While the endothelium does produce some factor VIII, the majority of factor VIII is produced in the liver.Robbins Basic Pathology 9th Edition, Chapt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base Pairs
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA and RNA. Dictated by specific hydrogen bonding patterns, "Watson–Crick" (or "Watson–Crick–Franklin") base pairs ( guanine– cytosine and adenine– thymine) allow the DNA helix to maintain a regular helical structure that is subtly dependent on its nucleotide sequence. The complementary nature of this based-paired structure provides a redundant copy of the genetic information encoded within each strand of DNA. The regular structure and data redundancy provided by the DNA double helix make DNA well suited to the storage of genetic information, while base-pairing between DNA and incoming nucleotides provides the mechanism through which DNA polymerase replicates DNA and RNA polymerase transcribes DNA into RNA. Many DNA-binding p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
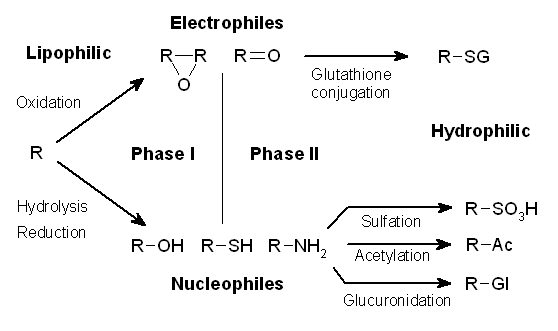
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is called pharmacokinetics. The metabolism of pharmaceutical drugs is an important aspect of pharmacology and medicine. For example, the rate of metabolism determines the duration and intensity of a drug's pharmacologic action. Drug metabolism also affects ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |