|
Pueraria Candollei Var. Mirifica
''Pueraria mirifica'', also known as กวาวเครือ Kwao Krua (among other names), is a plant found in northern and north eastern Thailand and Myanmar. In Thailand, the plant is known as "Kwao Krua Kao", the 'Kao' meaning white which distinguishes ''Pueraria mirifica'' from other plants with tuberous roots also sharing the 'Kwao Krua' designation such as ''Butea superba'', commonly called Kwao Krua Deng (Red) and the 'black' and 'dull grey' Kwao Krua plants. The species was definitively identified as ''Pueraria mirifica'' in 1952. Dried and powdered, the tuberous root of ''Pueraria mirifica'' has a history of domestic consumption in Thailand in traditional folk medicine as a rejuvenating herb to promote youthfulness in both women and men and is used widely within the now government-regulated practice of traditional Thai medicine. History Evidence of the use of ''Pueraria mirifica'' can be definitively identified as early as the 13th Century AD. The ancient capit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Herbert Kenneth Airy Shaw
Herbert Kenneth Airy Shaw (7 April 1902 – 19 August 1985) was a notable English botanist and classicist. He worked at Kew Gardens, as was an expert on tropical Asian botany and on entomology. The genus '' Airyantha'' is named for him. Life Airy Shaw was born at The Mount, Grange Road, Woodbridge, Suffolk to a father serving as Second Master at the Woodbridge Grammar School and a mother descended from George Biddell Airy, Astronomer Royal (1835–1881). His younger sister was the illustrator Margaret Olive Milne-Redhead (also known as Olive Shaw). In 1921 he entered Corpus Christi College, Cambridge University, to read classics, but he switched to natural sciences, taking his degree in 1924 and finishing in 1925, having been a student of Humphrey Gilbert-Carter. He was an active member of the Cambridge Inter-Collegiate Christian Union. Research Airy Shaw took a position at Kew Gardens after graduation, beginning as an unpaid volunteer in 1925. Initially he worked with Wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genistein
Genistein (C15H10O5) is a plant-derived, aglycone isoflavone. Genistein has the highest content of all isoflavones in soybeans and soy products, such as tempeh. As a type of phytoestrogen, genistein has estrogenic activity in vitro; consequently, its long-term intake by consuming soy products may affect reproductive organs, such as the uterus and breast. It was first isolated in 1899 from the dyer's broom, ''Genista tinctoria''; hence, the chemical name. The compound structure was established in 1926, when it was found to be identical with that of prunetol. It was chemically synthesized in 1928. Genistein is a primary secondary metabolite of the ''Trifolium'' species and '' Glycine max'' (soy). Natural occurrences Isoflavones, such as genistein and daidzein, occur in soybeans and various other plants, including lupin, fava beans, kudzu, psoralea, '' Flemingia vestita'', and coffee. It is present in red clover. In soybean products Isoflavone intake from consuming soy pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are toxic metals, toxic chemicals, microbe neurotoxins, radiation particles and even specific neurotransmitters when the system is out of balance. Also some types of drugs, e.g alcohol, and some venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'') are toxic to cells. Cell physiology Treating cells with the cytotoxic compound can result in a variety of prognoses. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
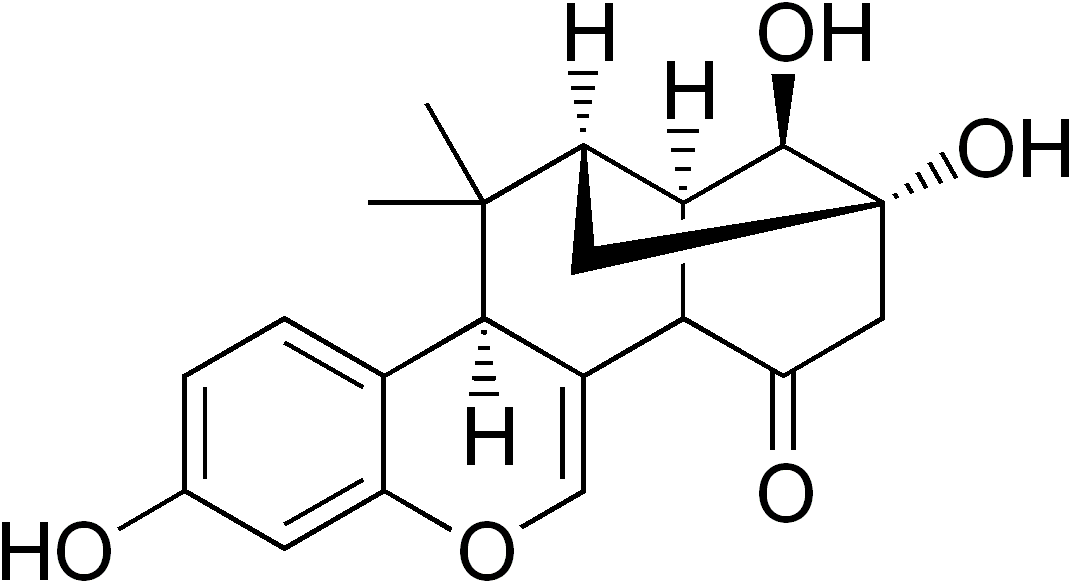
Miroestrol
Miroestrol is a phytoestrogen, a plant-derived chemical that mimics the biological activity of the hormone estrogen. Miroestrol was first reportedly isolated from the Thai herb '' Pueraria mirifica'' in 1960 and thought to be responsible for the supposed rejuvenating properties of the plant. However, more recent studies have suggested that the active ingredient may actually be the closely related chemical compound deoxymiroestrol (shown below), and the reported presence of miroestrol may only have been an artifact of the isolation procedure. When deoxymiroestrol is exposed to the oxygen in air, it is converted to miroestrol. A comparative study of the estrogenic properties of phytoestrogens found that both deoxymiroestrol and miroestrol were comparable in activity ''in vitro'' to other known phytoestrogens such as coumestrol as 17β-oestradiol agonists. Because of their estrogenic activities, miroestrol, deoxymiroestrol, and other related compounds have been the targets of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campesterol
Campesterol is a phytosterol whose chemical structure is similar to that of cholesterol, and is one of the ingredients for E number E499. Natural occurrences Many vegetables, fruits, nuts, and seeds contain campesterol, but in low concentrations. Banana, pomegranate, pepper, coffee, grapefruit, cucumber, onion, oat, potato, and lemon grass (citronella) are few examples of common sources containing campesterol at roughly 1–7 mg/100 g of the edible portion. In contrast, canola and corn oils contain as much as 16–100 mg/100 g. Levels are variable and are influenced by geography and growing environment. In addition, different strains have different levels of plant sterols. A number of new genetic strains are currently being engineered with the goal of producing varieties high in campesterol and other plant sterols. It is also found in dandelion coffee. It is so named because it was first isolated from the rapeseed (''Brassica campestris''). Precursor o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stigmasterol
Stigmasterol – a plant sterol (''phytosterol'') – is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes. In the European Union, it is a food additive listed with E number E499, and may be used in food manufacturing to increase the phytosterol content, potentially lowering the levels of LDL cholesterol. Discovery Once called ''Wulzen factor'' in the mid-20th century, stigmasterol was discovered by the University of California physiologist Rosalind Wulzen (born 1886). Natural occurrences Stigmasterol is an unsaturated phytosterol occurring in the plant fats or oils of numerous plants, such as soybean, calabar bean, and rape seed, and in herbs used in herbalism practices, including the Chinese herbs '' Ophiopogon japonicus'' (Mai men dong), in '' Mirabilis jalapa''. Stigmasterol is a constituent of various vegetables, legumes, nuts, seeds, and unpasteurized milk. Pasteurization will inactiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumestrol
Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana (a type of chick pea), and Alfalfa sprouts. Coumestrol is a phytoestrogen, mimicking the biological activity of estrogens. Phytoestrogens are able to pass through cell membranes due to their low molecular weight and stable structure, and they are able to interact with the enzymes and receptors of cells. Coumestrol binds to the ERα and ERβ with similar affinity to that of estradiol (94% and 185% of the relative binding affinity of estradiol at the ERα and ERβ, respectively), although the estrogenic activity of coumestro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genistin
Genistin is an isoflavone found in a number of dietary plants like soy and kudzu. It was first isolated in 1931 from the 90% methanol extract of a soybean meal, when it was found that hydrolysis with hydrochloric acid produced 1 mole (unit), mole each of genistein and glucose. Chemically it is the 7-O-beta-D-glucoside form of genistein and is the predominant form of the isoflavone naturally occurring in plants. In fact, studies in the 1970s revealed that 99% of the isoflavonoid, isoflavonoid compounds in soy are present as their glucosides. The glucosides are converted by digestive enzymes in the digestive system to exert their biological effects. Genistin is also converted to a more familiar genistein, thus, the biological activities including antiatherosclerotic, estrogenic and anticancer effects are analogous. Metabolism When ingested along the diet, genistin is readily converted to its aglycone form, genistein. It is hydrolysis, hydrolyzed by removing the covalent bond, covale ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |