|
Protomer (structural Biology)
In structural biology, a protomer is the structural unit of an Protein quaternary structure, oligomeric protein. It is the smallest unit composed of at least one protein chain. The protomers associate to form a larger Protein quaternary structure, oligomer of two or more copies of this unit. Protomers usually arrange in cyclic symmetry to form closed point group symmetries. The term was introduced by Chetverin to make nomenclature in the Na+/K+-ATPase, Na/K-ATPase enzyme unambiguous. This enzyme is composed of two subunits: a large, catalytic α subunit, and a smaller glycoprotein β subunit (plus a proteolipid, called γ-subunit). At the time it was unclear how many of each work together. In addition, when people spoke of a dimer (chemistry), dimer, it was unclear whether they were referring to αβ or to (αβ)2. Chetverin suggested to call αβ a protomer and (αβ)2 a diprotomer. Thus, in the work by Chetverin the term protomer was only applied to a Protein quaternary structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Biology
Structural biology deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes. In the 20th century, a variety of experimental techniques were developed to examine the 3D structures of biological molecules. The most prominent techniques are X-ray crystallography, nuclear magnetic resonance, and Electron microscope, electron microscopy. Through the discovery of X-ray, X-rays and its applications to protein crystals, structural biology was revolutionized, as now scientists could obtain the three-dimensional structures of biological molecules in atomic detail. Likewise, Nuclear magnetic resonance spectroscopy, NMR spectroscopy allowed information about protein structure and dynamic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-aminobenzoic Acid
4-Aminobenzoic acid (also known as ''para''-aminobenzoic acid or PABA because the two functional groups are attached to the benzene ring across from one another in the ''para'' position) is an organic compound with the formula H2NC6H4CO2H. PABA is a white crystalline solid, although commercial samples can appear gray. It is slightly soluble in water. It consists of a benzene ring substituted with amino and carboxyl groups. The compound occurs extensively in the natural world. Production and occurrence In industry, PABA is prepared mainly by two routes: * Reduction of 4-nitrobenzoic acid * Hoffman degradation of the monoamide derived from terephthalic acid. Food sources of PABA include liver, brewer's yeast (and unfiltered beer), kidney, molasses, mushrooms, and whole grains. Other food sources of PABA include spinach, liver, and oat seeds. Biology Biochemistry PABA is an intermediate in the synthesis of folate by bacteria, plants, and fungi. Many bacteria, including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a conditionally essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. The one-letter symbol Y was assigned to tyrosine for being alphabetically nearest of those letters available. Note that T was assigned to the structurally simpler threonine, U was avoided for its similarity with V for valine, W was assigned to tryptophan, while X was reserved for undetermined or atypical amino acids. The mnemonic t''Y''rosine was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodimer
In biochemistry, a protein dimer is a macromolecular complex or protein multimer, multimer formed by two protein monomers, or single proteins, which are usually Non-covalent interaction, non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", ''wikt:di-#Prefix, di-'' + ''wikt:-mer#Suffix, -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein IKBKG, NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HIV-1 Protease
HIV-1 protease or PR is a retroviral aspartyl protease (retropepsin), an enzyme involved with peptide bond hydrolysis in retroviruses, that is essential for the life-cycle of HIV, the retrovirus that causes AIDS. HIV-1 PR cleaves newly synthesized polyproteins (namely, Group-specific antigen, Gag and Gag-Pol (HIV), Pol) at nine cleavage sites to create the mature protein components of an HIV virion, the infectious form of a virus outside of the host cell. Without effective HIV-1 PR, HIV virions remain uninfectious. Structure Mature HIV protease exists as a 22 kDa homodimer, with each subunit made up of 99 amino acids. A single active site lies between the identical subunits and has the characteristic Aspartic acid, Asp-Threonine, Thr-Glycine, Gly (Asp25, Thr26 and Gly27) catalytic triad sequence common to aspartic proteases. As HIV-1 PR can only function as a dimer, the mature protease contains two Asp25 amino acids, one from each monomer, that act in conjunction with each ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
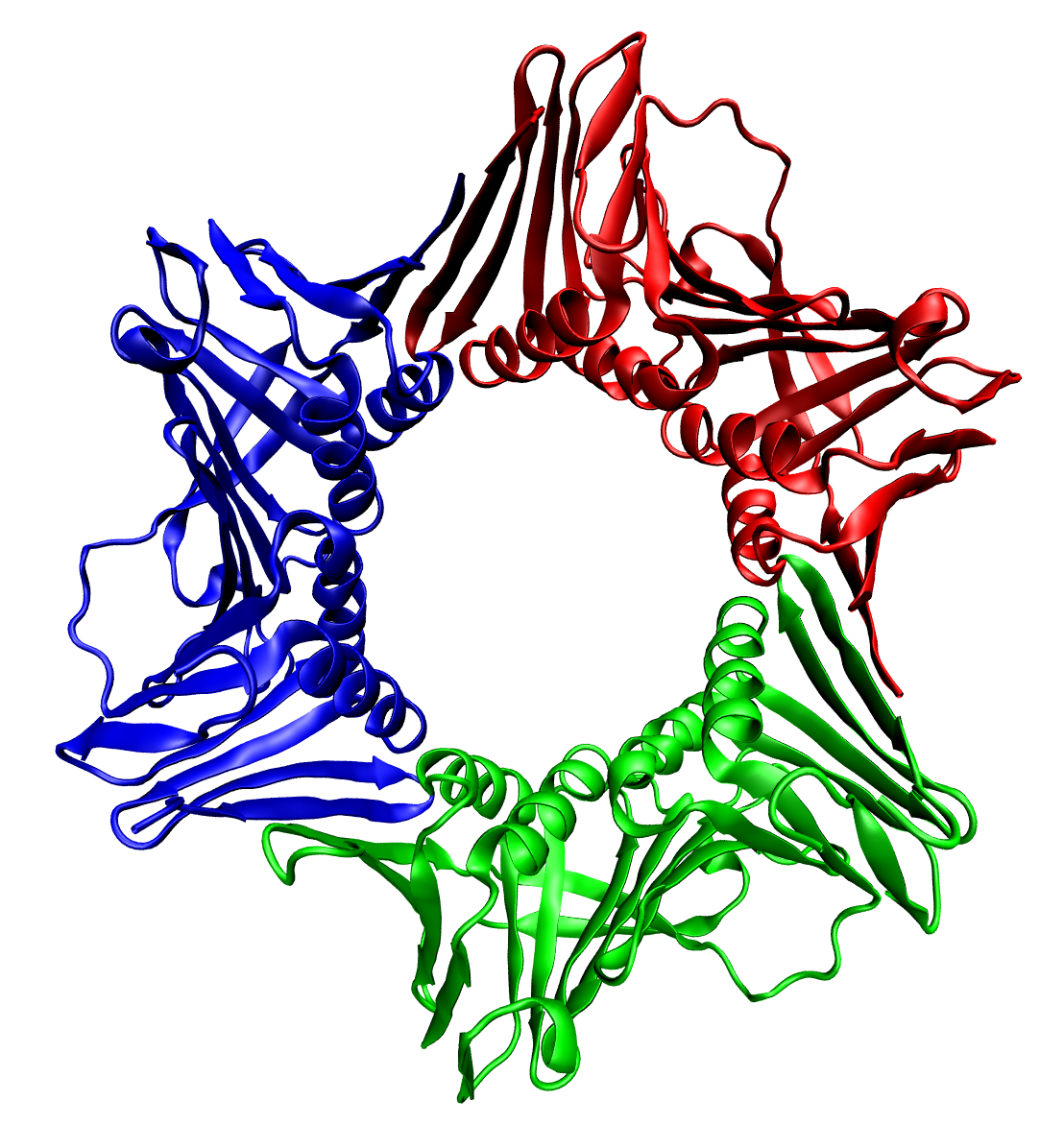
Capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The proteins making up the capsid are called capsid proteins or viral coat proteins (VCP). The virus genomic component inside the capsid, along with occasionally present virus core protein, is called the virus core. The capsid and core together are referred to as a nucleocapsid (cf. also virion). Capsids are broadly classified according to their structure. The majority of the viruses have capsids with either helical or icosahedral structure. Some viruses, such as bacteriophages, have developed more complicated structures due to constraints of elasticity and electrostatics. The icosahedral shape, which has 20 equilateral triangular faces, approximates a sphere, while th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate Carbamoyltransferase
Aspartate carbamoyltransferase (also known as aspartate transcarbamoylase or ATCase) catalyzes the first step in the pyrimidine biosynthetic pathway (). In '' E. coli'', the enzyme is a multi- subunit protein complex composed of 12 subunits (300 kDa in total). The composition of the subunits is C6R6, forming 2 trimers of catalytic subunits (34 kDa) and 3 dimers of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates. The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions. ATCase does not follow Michaelis–Menten kinetics. Instead, it lies between its low-activity, low-affinity "tense" and its high-activity, high-affinity "relaxed" states. The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterotetramer
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
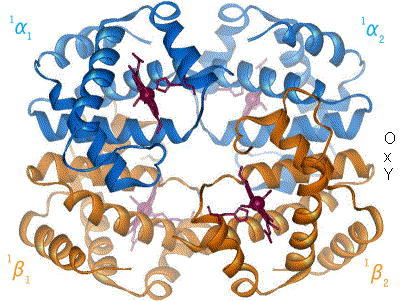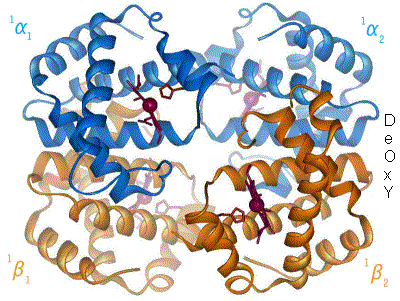
Hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers an animal's metabolism. A healthy human has 12to 20grams of hemoglobin in every 100mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and a globulin. In mammals, hemoglobin makes up about 96% of a red blood cell's dry matter, dry weight (excluding water), and around 35% of the total weight (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL of O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood plasma alone. The mammalian hemoglobin molecule can bind and transport up to four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Quaternary Structure
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It includes organizations from simple dimers to large homooligomers and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complexes of proteins with nucleic acids and other cofactors. Description and examples Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of the protein subunits w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |