|
Pressure Melting Point
The pressure melting point of ice is the temperature at which ice melts at a given pressure. The pressure melting point is nearly a constant 0 °C at pressures above the triple point at 611.7 Pa—where ice, water, and water vapour coexist in equilibrium—through atmospheric pressure (100 kPa) until about 10 MPa. With increasing pressure above 10 MPa, the pressure melting point decreases to a minimum of −21.9 °C at 209.9 MPa. Thereafter, the pressure melting point rises rapidly with pressure, passing back through 0 °C at 632.4 MPa. Pressure melting point in glaciers Glaciers are subject to geothermal heat flux from below and atmospheric warming or cooling from above. As the pressure increases with depth in a glacier from the weight of the ice above, the pressure melting point of ice decreases within bounds, as shown in the diagram. The level where ice can start melting is where the pressure melting point equals the actual temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram Of Water
This page provides supplementary data to the article properties of water. Further comprehensive authoritative data can be found at the ''NIST Chemistry WebBook'' page on thermophysical properties of fluids. Structure and properties Thermodynamic properties Liquid physical properties Water/steam equilibrium properties Vapor pressure formula for steam in equilibrium with liquid water: : \log_ P = A - \frac, where ''P'' is equilibrium vapor pressure in k Pa, and ''T'' is temperature in kelvins. For ''T'' = 273 K to 333 K: ''A'' = 7.2326; ''B'' = 1750.286; ''C'' = 38.1. For ''T'' = 333 K to 423 K: ''A'' = 7.0917; ''B'' = 1668.21; ''C'' = 45.1. Data in the table above is given for water–steam equilibria at various temperatures over the entire temperature range at which liquid water can exist. Pressure of the equilibrium is given in the second column in k Pa. The third column is the heat content of each gram of the liquid phase relative to water at 0 °C. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degree Celsius
The degree Celsius is the unit of temperature on the Celsius temperature scale "Celsius temperature scale, also called centigrade temperature scale, scale based on 0 ° for the melting point of water and 100 ° for the boiling point of water at 1 atm pressure." (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the closely related Kelvin scale. The degree Celsius (symbol: °C) can refer to a specific point on the Celsius temperature scale or to a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who proposed the first version of it in 1742. The unit was called ''centigrade'' in several languages (from the Latin ''centum'', which means 100, and ''gradus'', which means steps) for many years. In 1948, the International Committee for Weights and Measures renamed it to honor Celsius and also to remove ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three Phase (matter), phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sublimation (phase transition), sublimation, Melting, fusion, and vaporisation curves meet. For example, the triple point of Mercury (element), mercury occurs at a temperature of and a pressure of 0.165 Milli, mPascal (unit), Pa. In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphism (materials science), polymorphs. Helium-4 is unusual in that it has no sublimation/deposition curve and therefore no triple points where its solid phase meets its gas phase. Instead, it has a vapor-liquid-superfluid point, a solid-liquid-superfluid point, a solid-solid-liquid point, and a solid-solid-superfluid point. None of these should be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pascal (unit)
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI). It is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is an SI coherent derived unit defined as one newton per square metre (N/m2). It is also equivalent to 10 barye (10 Ba) in the CGS system. Common multiple units of the pascal are the hectopascal (1 hPa = 100 Pa), which is equal to one millibar, and the kilopascal (1 kPa = 1000 Pa), which is equal to one centibar. The unit of measurement called '' standard atmosphere (atm)'' is defined as . Meteorological observations typically report atmospheric pressure in hectopascals per the recommendation of the World Meteorological Organization, thus a standard atmosphere (atm) or typical sea-level air pressure is about 1013 hPa. Reports in the United States typically use inches of mercury or millibars (hectopascals). In Cana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glaciers
A glacier (; or ) is a persistent body of dense ice, a form of rock, that is constantly moving downhill under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires distinguishing features, such as crevasses and seracs, as it slowly flows and deforms under stresses induced by its weight. As it moves, it abrades rock and debris from its substrate to create landforms such as cirques, moraines, or fjords. Although a glacier may flow into a body of water, it forms only on land“Glacier, N., Pronunciation.” Oxford English Dictionary, Oxford UP, June 2024, https://doi.org/10.1093/OED/7553486115. Accessed 25 Jan. 2025. and is distinct from the much thinner sea ice and lake ice that form on the surface of bodies of water. On Earth, 99% of glacial ice is contained within vast ice sheets (also known as "continental glaciers") in the polar regions, but glaciers may be found in mountain ranges on every c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Shelf
An ice shelf is a large platform of glacial ice floating on the ocean, fed by one or multiple tributary glaciers. Ice shelves form along coastlines where the ice thickness is insufficient to displace the more dense surrounding ocean water. The boundary between the ice shelf (floating) and grounded ice (resting on bedrock or sediment) is referred to as the grounding line; the boundary between the ice shelf and the open ocean (often covered by sea ice) is the ice front or calving front. Ice shelves are found in Antarctica and the Arctic (Greenland, Northern Canada, and the Russian Arctic), and can range in thickness from about . The world's largest ice shelves are the Ross Ice Shelf and the Filchner-Ronne Ice Shelf in Antarctica. The movement of ice shelves is principally driven by gravity-induced pressure from the grounded ice. That flow continually moves ice from the grounding line to the seaward front of the shelf. Typically, a shelf front will extend forward for years ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
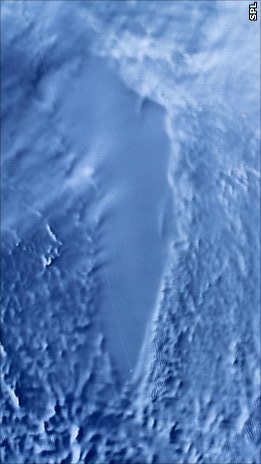
Subglacial Lake
A subglacial lake is a lake that is found under a glacier, typically beneath an ice cap or ice sheet. Subglacial lakes form at the boundary between ice and the underlying bedrock, where liquid water can exist above the lower melting point of ice under high pressure. Over time, the overlying ice gradually melts at a rate of a few millimeters per year. Meltwater flows from regions of high to low hydraulic pressure under the ice and pools, creating a body of liquid water that can be isolated from the external environment for millions of years. Since the first discoveries of subglacial lakes under the Antarctic Ice Sheet, more than 400 subglacial lakes have been discovered in Antarctica, beneath the Greenland Ice Sheet, and under Iceland's Vatnajökull ice cap. Subglacial lakes contain a substantial proportion of Earth's liquid freshwater, with the volume of Antarctic subglacial lakes alone estimated to be about 10,000 km3, or about 15% of all liquid freshwater on Earth. As ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |