|
Pregnancy Zone Protein
Pregnancy zone protein (PZP), also known as the pregnancy-associated α2-glycoprotein (α2-PAG or PAα2G), is a protein which in humans is encoded by the ''PZP'' gene on chromosome 12. PZP is part of the alpha-2 globulin family of proteins. It is often associated with pregnancy, during which it can be the most abundant among the plasma proteins.Chiabrando GA, Vides MA, Sanchez MC (2002). “Differential binding properties of human pregnancy zone protein– and a2-macroglobulin–proteinase complexes to low-density lipoprotein receptor-related protein”. ''Archives of Biochemistry and Biophysics.'' 398(1): 73–78. PZP is believed to play a role in immune-regulation during pregnancy, however many aspects of its mechanism, function and structure are yet to be determined.Oh JW, Kim SK, Cho K-C, Kim M-S, Suh CS, Lee JR, Kim KP (2017). “Proteomic analysis of human follicular fluid in poor ovarian responders during in vitro fertilization”.''Proteomics.'' 17(6). Charkoftaki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. Methionine is also involved in angiogenesis and various processes related to DNA transcription, epigenetic expression, and gene regulation. Methionine was first isolated in 1921 by John Howard Mueller. It is Genetic code, encoded by the codon AUG. It was named by Satoru Odake in 1925, as an abbreviation of its structural description 2-amino-4-(methylthio)butanoic acid. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the proton ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Placental Growth Factor
Placental growth factor (PlGF) is a protein that in humans is encoded by the ''PGF'' gene. Placental growth factor is a member of the VEGF (vascular endothelial growth factor) sub-family - a key molecule in angiogenesis and vasculogenesis, in particular during embryogenesis. The main source of PlGF during pregnancy is the placental trophoblast. ''PGF'' is also expressed in many other tissues, including the villous trophoblast. The placental growth factor gene (''PGF'') is a protein-coding gene and a member of the vascular endothelial growth factor (VEGF) family. ''PGF'' is expressed only in human umbilical vein endothelial cells, and the placenta. PGF is ultimately associated with angiogenesis. Specifically, PGF plays a role in trophoblast growth and differentiation. Trophoblast cells, specifically extravillous trophoblast cells, are responsible for invading the uterine wall and the maternal spiral arteries. The extravillous trophoblast cells produce a blood vessel of lar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macromolecule
A macromolecule is a "molecule of high relative molecular mass, the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass." Polymers are physical examples of macromolecules. Common macromolecules are biopolymers (nucleic acids, proteins, and carbohydrates). and polyolefins (polyethylene) and polyamides (nylon). Synthetic macromolecules Many macromolecules are synthetic polymers (plastics, synthetic fibers, and synthetic rubber. Polyethylene is produced on a particularly large scale such that ethylene is the primary product in the chemical industry. Macromolecules in nature * Proteins are polymers of amino acids joined by peptide bonds. * DNA and RNA are polymers of nucleotides joined by phosphodiester bonds. These nucleotides consist of a phosphate group, a sugar ( ribose in the case of RNA, deoxyribose in the case of DNA), and a nucleotide base (either adenine, guan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasminogen Activator
Plasmin is an important enzyme () present in blood that degrades many blood plasma proteins, including fibrin clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein (in the zymogen form of plasminogen) is encoded by the ''PLG'' gene. Function Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. Plasmin is also integrally involved in inflammation. It cleaves fibrin, fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsin, belongs to the family of serine proteases. Plasmin is released as a zymogen called plasminogen (PLG) from the liver into the systemic circulation. Two major glycoforms of plasminogen are present in humans - type I plasminogen contains two glycos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytokine
Cytokines () are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are produced by a broad range of cells, including immune cells like macrophages, B cell, B lymphocytes, T cell, T lymphocytes and mast cells, as well as Endothelium, endothelial cells, fibroblasts, and various stromal cells; a given cytokine may be produced by more than one type of cell. Due to their size, cytokines cannot cross the lipid bilayer of cells to enter the cytoplasm and therefore typically exert their functions by interacting with specific cytokine receptor, cytokine receptors on the target cell surface. Cytokines are especially important in the immune system; cytokines modulate the balance between humoral immunity, humoral and cell-mediated immunity, cell-based immune responses, and they regulate the maturation, growth, and responsiveness of particular cell populations. Some cytokines enhance or inhibit the action of other cytokines in complex way ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
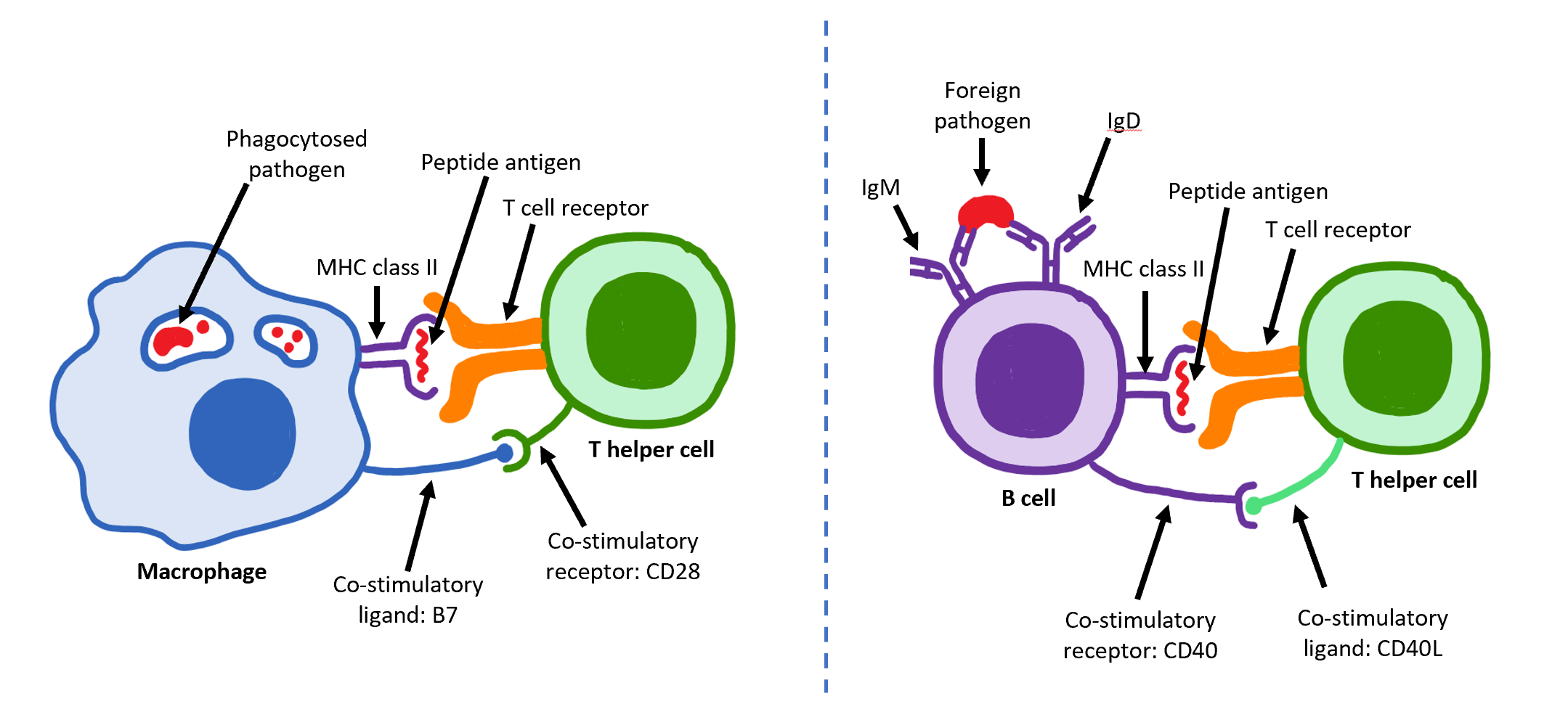
T Helper Cell
The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell Immunoglobulin class switching, antibody class switching, breaking Cross-presentation, cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases. Structure and function Th cells contain and release cytokines to aid other immune cells. Cytokines are small protein mediators that alter the behavior of target cells that express Receptor (biochemistry), receptors for those cytokines. These cells help polar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease Inhibitor (biology)
In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases (enzymes that aid proteolysis, the breakdown of proteins). Many naturally occurring protease inhibitors are proteins. In medicine, ''protease inhibitor'' is often used interchangeably with alpha 1-antitrypsin (A1AT, which is abbreviated PI for this reason). A1AT is indeed the protease inhibitor most often involved in disease, namely in alpha-1 antitrypsin deficiency. Classification Protease inhibitors may be classified either by the type of protease they inhibit, or by their mechanism of action. In 2004 Rawlings and colleagues introduced a classification of protease inhibitors based on similarities detectable at the level of amino acid sequence. This classification initially identified 48 families of inhibitors that could be grouped into 26 related superfamily (or clans) by their structure. According to the MEROPS database there are now 81 families of inhibito ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-2-Macroglobulin
α2-Macroglobulin (α2M) or alpha-2-macroglobulin is a large (720 KDa) plasma protein found in the blood. It is mainly produced by the liver, and also locally synthesized by macrophages, fibroblasts, and adrenocortical cells. In humans it is encoded by the ''A2M'' gene. α2-Macroglobulin acts as an antiprotease and is able to inactivate an enormous variety of proteinases. It functions as an inhibitor of fibrinolysis by inhibiting plasmin and kallikrein. It functions as an inhibitor of coagulation by inhibiting thrombin. α2-macroglobulin may act as a carrier protein because it also binds to numerous growth factors and cytokines, such as platelet-derived growth factor, basic fibroblast growth factor, TGF-β, insulin, and IL-1β. No specific deficiency with associated disease has been recognized, and no disease state is attributed to low concentrations of α2-macroglobulin. The concentration of α2-macroglobulin rises 10-fold or more in the nephrotic syndrome when other lower mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-linked Glycosylation
''N''-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), in a process called ''N''-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of ''N''-linked glycans. Energetics of bond formation There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |