|
Potassium Hydrogen Iodate
An iodate is the polyatomic anion with the formula . It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. Iodate salts are often colorless. They are the salts of iodic acid. Structure Iodate is pyramidal in structure. The O–I–O angles range from 97° to 105°, somewhat smaller than the O–Cl–O angles in chlorate. Reactions Redox Iodate is one of several oxyanions of iodine, and has an oxidation number of +5. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate. Iodate is reduced by sulfite: : Iodate oxidizes iodide in acidic conditions: : Similarly, chlorate oxidizes iodide to iodate: : Iodate is also obtained by reducing a periodate with a sulfide. The byproduct of the reaction is a sulfoxide. Acid-base Iodate is unusual in that it forms a strong hydrogen bond with its parent acid: : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Space-filling Model
In chemistry, a space-filling model, also known as a ''calotte model'', is a type of three-dimensional (3D) molecular model where the atoms are represented by spheres whose radii are proportional to the radii of the atoms and whose center-to-center distances are proportional to the distances between the atomic nuclei, all in the same scale. Atoms of different chemical elements are usually represented by spheres of different colors. Space-filling calotte models are also referred to as CPK models after the chemists Robert Corey, Linus Pauling, and Walter Koltun, who over a span of time developed the modeling concept into a useful form. They are distinguished from other 3D representations, such as the ball-and-stick and skeletal models, by the use of the "full size" space-filling spheres for the atoms. The models are tactile and manually rotatable. They are useful for visualizing the effective shape and relative dimensions of a molecule, and (because of the rotatability) the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroiodate
A fluorooxoiodate or fluoroiodate is a chemical compound or ion derived from iodate, by substituting some of the oxygen by fluorine. They have iodine in the +5 oxidation state. The iodine atoms have a stereochemically active lone-pair of electrons. Many are non-centrosymmetric, and are second harmonic generators (SHG) of intense light shining through them. They are under investigation as materials for non-linear optics In mathematics and science, a nonlinear system (or a non-linear system) is a system in which the change of the output is not proportional to the change of the input. Nonlinear problems are of interest to engineers, biologists, physicists, mathem ..., such as for generating ultraviolet light from visible or infrared lasers. Different ions include OF4sup>−, O2F2sup>−, O3Fsup>2−, and 2O5F2sup>2−. They are distinct from the iodate fluorides which are mixed anion compounds that do not have fluorine-iodine bonds. Properties Fluoroiodates are transparent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Salt
A double salt is a salt that contains two or more different cations or anions. Examples of double salts include alums (with the general formula ) and Tutton's salts (with the general formula ). Other examples include potassium sodium tartrate, ammonium iron(II) sulfate (Mohr's salt), potassium uranyl sulfate (used to discover radioactivity) and bromlite . The fluorocarbonates contain fluoride and carbonate anions. Many coordination complexes form double salts. Double salts should not be confused with complexes. Double salts only exist in the solid. When dissolved in water, a double salt acts as a mixture of the two separate salts: it completely dissociates into simple ions while a hexaaquo complex does not; the complex ion remains unchanged. Similarly, potassium hexaiodoytterbate(II) is a complex salt and contains the discrete hexaiodoytterbate(II) ion , which remains intact in aqueous solution An aqueous solution is a solution in which the solvent is water. It is mostly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulletin Of The World Health Organization
The ''Bulletin of the World Health Organization'' is a monthly public health journal published by the World Health Organization that was established in 1948. Articles are published in English and abstracts are available in Arabic, Chinese, English, French, Russian, and Spanish. According to the ''Journal Citation Reports'', the journal has a 2022 impact factor of 11.1, ranking it 12th out of 207 journals in the category "Public, Environmental & Occupational Health". It is open access journal and uses the Creative Commons 3.0 IGO license (specifically CC BY 3.0 IGO). History The first issue of the ''Bulletin of the World Health Organization'' was published in 1948 under the initiative of the Interim Commission, and has been published monthly ever since. Abstracting and indexing The Bulletin is abstracted and indexed in Abstracts on Hygiene, Biological Abstracts, Current Contents, Excerpta Medica, International Pharmaceutical Abstracts, Index Medicus/MEDLINE/PubMed, Nutrition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
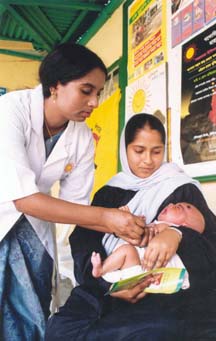
Iodized Salt
Iodised salt ( also spelled iodized salt) is table salt mixed with a minute amount of various iodine salts. The ingestion of iodine prevents iodine deficiency. Worldwide, iodine deficiency affects about two billion people and is the leading preventable cause of intellectual and developmental disabilities. Deficiency also causes thyroid gland problems, including endemic goitre. In many countries, iodine deficiency is a major public health problem that can be cheaply addressed by purposely adding small amounts of iodine to the sodium chloride salt. Iodine is a micronutrient and dietary mineral that is naturally present in the food supply in some regions, especially near sea coasts but is generally quite rare in the Earth's crust since iodine is a so-called heavy element, and abundance of chemical elements typically declines with greater atomic mass. Where natural levels of iodine in the soil are low and vegetables do not take up the iodine, iodine added to salt provides the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
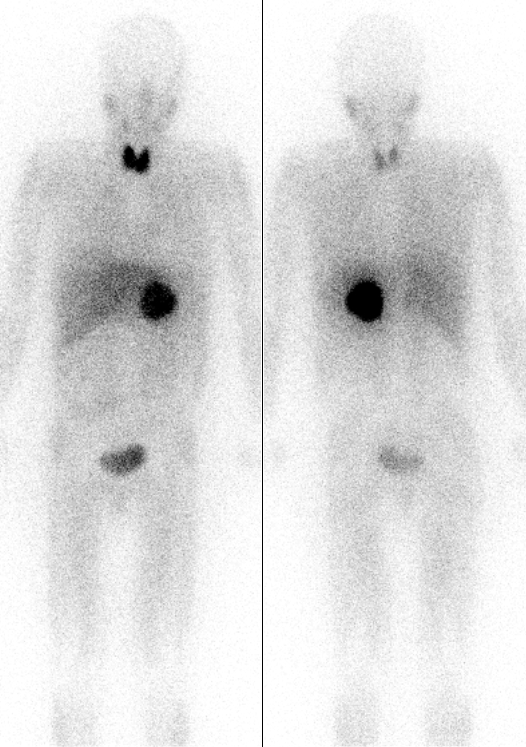
Radioiodine
There are 40 known isotopes of iodine (53I) from 108I to 147I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element. Its longest-lived radioactive isotope, 129I, has a half-life of 16.14 million years, which is too short for it to exist as a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents. All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine - 123I, 124I, 125I, and 131I. All industrial use of radioactive iodine isotopes involves these four. The isotope 135I has a half-life less than seven hours, which is inconveniently sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prophylaxis
Preventive healthcare, or prophylaxis, is the application of healthcare measures to prevent diseases.Hugh R. Leavell and E. Gurney Clark as "the science and art of preventing disease, prolonging life, and promoting physical and mental health and efficiency. Leavell, H. R., & Clark, E. G. (1979). Preventive Medicine for the Doctor in his Community (3rd ed.). Huntington, NY: Robert E. Krieger Publishing Company. Disease and disability are affected by environmental factors, genetic predisposition, disease agents, and lifestyle choices, and are dynamic processes that begin before individuals realize they are affected. Disease prevention relies on anticipatory actions that can be categorized as primal, primary, secondary, and tertiary prevention. Each year, millions of people die of preventable causes. A 2004 study showed that about half of all deaths in the United States in 2000 were due to preventable behaviors and exposures. Leading causes included cardiovascular disease, chron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used. It is also used for treating skin sporotrichosis and phycomycosis. It is a supplement used by people with low dietary intake of iodine. It is administered orally. Common side effects include vomiting, diarrhea, abdominal pain, rash, and swelling of the salivary glands. Other side effects include allergic reactions, headache, goitre, and depression. While use during pregnancy may harm the baby, its use is still recommended in radiation emergencies. Potassium iodide has the chemical formula K I. Commercially it is made by mixing potassium hydroxide with iodine. Potassium iodide has been used medically since at least 1820. It is on the World Health Organization's List of Essential Medicines. Potassium iodide is available as a g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Iodate
Potassium iodate ( K I O3) is an ionic inorganic compound with the formula . It is a white salt that is soluble in water. Preparation and properties It can be prepared by reacting a potassium-containing base such as potassium hydroxide with iodic acid, for example: : HIO3 + KOH → KIO3 + H2O It can also be prepared by adding iodine to a hot, concentrated solution of potassium hydroxide: :3 I2 + 6 KOH → KIO3 + 5 KI + 3 H2O Or by fusing potassium iodide with potassium chlorate, bromate or perchlorate, the melt is extracted with water and potassium iodate is isolated from the solution by crystallization: :KI + KClO3 → KIO3 + KCl The analogous reaction with potassium hypochlorite is also possible:KI + 3KOCl → 3KCl + KIO3Conditions/substances to avoid include: heat, shock, friction, combustible materials, reducing materials, aluminium, organic compounds, carbon, hydrogen peroxide and sulfides. Applications Potassium iodate is sometimes used for iodination of tabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Iodate
Calcium iodate is any of two inorganic compounds with the formula Ca(IO3)2(H2O)x, where x = 0 or 1. Both are colourless salts that occur as the minerals lautarite and bruggenite, respectively. A third mineral form of calcium iodate is dietzeite, a salt containing chromate with the formula Ca2(IO3)2CrO4. These minerals are the most common compounds containing iodate. Production and uses Lautarite, described as ''the'' most important mineral source of iodine, is mined in the Atacama Desert. Processing of the ore entails reduction of its aqueous extracts with sodium bisulfite to give sodium iodide. This comproportionation reaction is a major source of the sodium iodide. Calcium iodate can be produced by the anodic oxidation of calcium iodide or by passing chlorine into a hot solution of lime in which iodine has been dissolved. Calcium iodate is used as an iodine supplement in chicken feed. Ethylenediamine dihydroiodide (EDDI) is a more typical source of nutritional iodi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |