|
Polymorphic Toxins
Polymorphic toxins (PTs) are multi-domain proteins primarily involved in competition between bacteria but also involved in pathogenesis when injected in eukaryotic cells. They are found in all major bacterial clades. Bacteria live in complex multispecies communities such as biofilms and human-associated microbiotas. The dynamics and structure of these communities are greatly influenced by interbacterial competition through the secretion of toxic effectors. Bacteria have evolved several systems to outcompete their neighbors by poisoning them through a contact-dependent killing (including effectors of type V and VI secretion systems) or the release of soluble toxins (including colicins) in the environment. Definition Polymorphic toxins are bacterial exotoxins which share common features regarding their domain architecture. Each family of PTs is defined by a conserved N-terminal region associated with diverse C-terminal (CT) toxic domains, which can be found in several other PT fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
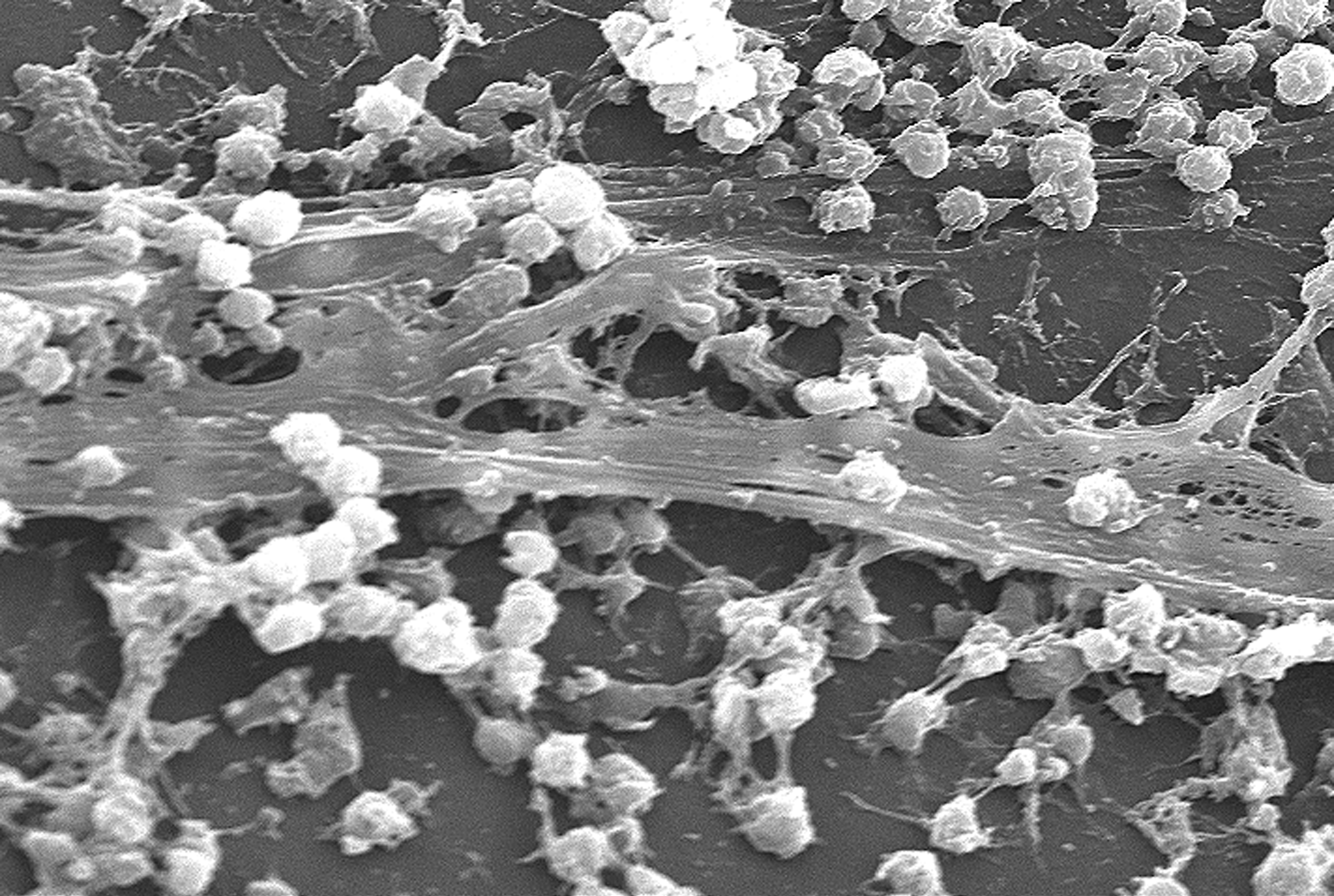
Biofilm
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes". Biofilms may form on living or non-living surfaces and can be prevalent in natural, industrial, and hospital settings. They may constitute a microbiome or be a portion of it. The microbial cells growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which, by contrast, are single cells that may float or swim in a liquid medium. Biofilms can form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptidase
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MafB Toxins
MafB toxins are exotoxins secreted by pathogenic ''Neisseria'' species (including meningococcus and gonococcus ''Neisseria gonorrhoeae'', also known as ''gonococcus'' (singular), or ''gonococci'' (plural), is a species of Gram-negative diplococci bacteria isolated by Albert Ludwig Sigesmund Neisser, Albert Neisser in 1879. It causes the sexually transmit ...). MafB toxins belong to the category of polymorphic toxins. The N-terminal region of MafB proteins harbors a domain of unknown function nameDUF1020while the C-terminal region is variable and harbors a toxic domain. MafB toxins are involved in interbacterial competition. References External links * http://pfam.xfam.org/family/PF06255 Toxins {{med-toxic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hcp Toxins
HCP may refer to: Businesses * HashiCorp (NASDAQ: HCP), an American software company * Healthpeak Properties (formerly HCP, Inc.), an American investment company * H. Cegielski – Poznań, a Polish manufacturing company * Health Consumer Powerhouse, a Swedish think tank * Highland Capital Partners, a venture capital firm Health and medicine * Healthcare professional * Hereditary coproporphyria * Himalayan Cataract Project * Human Connectome Project * Hydrocephalus, or "water on the brain" * Haemolysin-coregulated protein, in the Type VI secretion system * Healthcare proxy Other uses * Aga Khan Historic Cities Programme * Habitat Conservation Plan * Hamiltonian Circuit Problem, in computer science * Handicap (horse racing), in horse racing * Hardened cement paste * Harding Charter Preparatory High School, in Oklahoma City, Oklahoma, United States * Haut Commissariat au Plan in Morocco * Heritage College, Perth, in Australia * Hexagonal close-packed arrangement of spheres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhs Toxins
Rhs toxins belong to the polymorphic toxin category of bacterial exotoxins. Rhs proteins are widespread and can be produced by both Gram-negative and Gram-positive bacteria. Rhs toxins are very large proteins of usually more than 1,500 aminoacids with variable C-terminal toxic domains. Their toxic activity can either target eukaryotes or other bacteria. Domain architecture In their large N-terminal region, Rhs toxins comprise RHS/YD repeats in various numberPF05593 (RHS meaning Rearrangement Hot Spot) and another "RHS-repeats associated core" domainPF03527. In contrast, their C-terminal regions are shorter and harbor highly variable C-terminal domains including many domains with a predicted nuclease activity. Function Anti-eukaryotic activity These toxins encompass Rhs toxins of insect pathogens with an activity against insects. This group also include Rhs toxins with an activity against human phagocytic cells that contribute to pathogenesis of ''Pseudomonas aeruginosa''. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colicin
A colicin is a type of bacteriocin produced by and toxic to some strains of ''Escherichia coli''. Colicins are released into the environment to reduce competition from other bacterial strains. Colicins bind to outer membrane receptors, using them to translocate to the cytoplasm or cytoplasmic membrane, where they exert their cytotoxic effect, including depolarisation of the cytoplasmic membrane, DNase activity, RNase activity, or inhibition of murein synthesis. Structure Channel-forming colicins (colicins A, B, E1, Ia, Ib, and N) are transmembrane proteins that depolarize the cytoplasmic membrane, leading to dissipation of cellular energy. These colicins contain at least three domains: an N-terminal translocation domain responsible for movement across the outer membrane and periplasmic space; a central domain responsible for receptor recognition; and a C-terminal cytotoxic domain responsible for channel formation in the cytoplasmic membrane. One domain regulates the target a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNase
Deoxyribonuclease (DNase, for short) refers to a group of glycoprotein endonucleases which are enzymes that catalyze the hydrolytic cleavage of phosphodiester linkages in the DNA backbone, thus degrading DNA. The role of the DNase enzyme in cells includes breaking down extracellular DNA (ecDNA) excreted by apoptosis, necrosis, and neutrophil extracellular traps (NET) of cells to help reduce inflammatory responses that otherwise are elicited. A wide variety of deoxyribonucleases are known and fall into one of two families (DNase I or DNase II), which differ in their substrate specificities, chemical mechanisms, and biological functions. Laboratory applications of DNase include purifying proteins when extracted from prokaryotic organisms. Additionally, DNase has been applied as a treatment for diseases that are caused by ecDNA in the blood plasma. Assays of DNase are emerging in the research field as well. Types The two main types of DNase found in metazoans are known as deox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
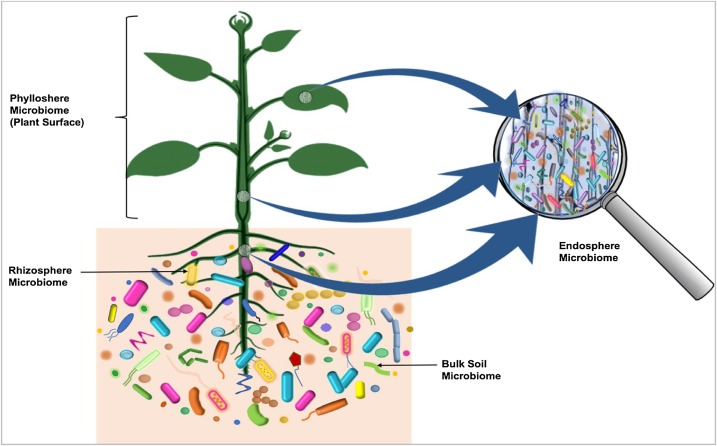
Microbiota
Microbiota are the range of microorganisms that may be commensal, symbiotic, or pathogenic found in and on all multicellular organisms, including plants. Microbiota include bacteria, archaea, protists, fungi, and viruses, and have been found to be crucial for immunologic, hormonal, and metabolic homeostasis of their host. The term ''microbiome'' describes either the collective genomes of the microbes that reside in an ecological niche or within the microbes themselves. The microbiome and host emerged during evolution as a synergistic unit from epigenetics and genetic characteristics, sometimes collectively referred to as a holobiont. The presence of microbiota in human and other metazoan guts has been critical for understanding the co-evolution between metazoans and bacteria. Microbiota play key roles in the intestinal immune and metabolic responses via their fermentation product ( short-chain fatty acid), acetate. Introduction All plants and animals, from simple l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RNase
Ribonuclease (commonly abbreviated RNase) is a type of nuclease that catalyzes the degradation of RNA into smaller components. Ribonucleases can be divided into endoribonucleases and exoribonucleases, and comprise several sub-classes within the EC 2.7 (for the phosphorolytic enzymes) and 3.1 (for the hydrolytic enzymes) classes of enzymes. Function All organisms studied contain many RNases of two different classes, showing that RNA degradation is a very ancient and important process. As well as clearing of cellular RNA that is no longer required, RNases play key roles in the maturation of all RNA molecules, both messenger RNAs that carry genetic material for making proteins and non-coding RNAs that function in varied cellular processes. In addition, active RNA degradation systems are the first defense against RNA viruses and provide the underlying machinery for more advanced cellular immune strategies such as RNAi. Some cells also secrete copious quantities of non-specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |