|
Polymerization Inhibitor
In polymer chemistry, polymerisation inhibitors (US: polymerization inhibitors) are chemical compounds added to monomers to prevent their self-polymerisation. Unsaturated monomers such as acrylates, vinyl chloride, butadiene and styrene require inhibitors for both processing and safe transport and storage. Many monomers are purified industrially by distillation, which can lead to thermally-initiated polymerisation. Styrene, for example, is distilled at temperatures above 100 °C whereupon it undergoes thermal polymerisation at a rate of ~2% per hour. This polymerisation is undesirable, as it can foul the fractionating tower; it is also typically exothermic, which can lead to a runaway reaction and potential explosion if left unchecked. Once initiated, polymerisation is typically radical in mechanism and as such many polymerisation inhibitors act as radical scavengers. Inhibitors vs retarders The term 'inhibitor' is often used in a general sense to describe any compound used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Schulz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion-controlled Reaction
Diffusion-controlled (or diffusion-limited) chemical reaction, reactions are reactions in which the reaction rate is equal to the rate of transport of the reactants through the reaction medium (usually a solution). The process of chemical reaction can be considered as involving the diffusion of reactants until they encounter each other in the right stoichiometry and form an activated complex which can form the product species. The observed rate of chemical reactions is, generally speaking, the rate of the slowest or "rate determining" step. In diffusion controlled reactions the formation of products from the activated complex is much faster than the diffusion of reactants and thus the rate is governed by collision frequency. Diffusion control is rare in the gas phase, where rates of diffusion of molecules are generally very high. Diffusion control is more likely in solution where diffusion of reactants is slower due to the greater number of collisions with solvent molecules. React ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
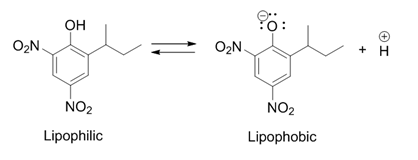
Dinoseb
Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity. It also finds use as a polymerisation inhibitor, where it is often referred to as DNBP. It is used to prevent the thermally induced polymerisation of styrene and other unsaturated monomers when they are being purified by distillation. History In 1892, dinitro-''ortho''-cresol (2,4-dinitro-6-methylphenol), a chemical compound closely related to dinoseb, was discovered in Germany and first used as an insecticide. It was later also used as an herbicide and also fungicide after those characteristics were discovered. In 1945 the ''ortho''-methyl group was replaced by a ''sec''-butyl group, producing dinoseb. This compound had a superior contact and stomach activity on insects and mites. Dinoseb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitro-ortho-cresol
Dinitro-''ortho''-cresol (DNOC) is an organic compound with the structural formula CH3C6H2(NO2)2OH. It is a yellow solid that is only slightly soluble in water. It is extremely toxic to humans and was previously used as a herbicide and insecticide. Preparation This compound is prepared by disulfonation of ''o''-cresol. The resulting disulfonate is then treated with nitric acid to give DNOC. A variety of related derivatives are known including those where the methyl group is replaced by ''sec''-butyl (dinoseb), ''tert''-butyl ( dinoterb), and 1-methylheptyl ( dinocap). These are prepared by the direct nitration of the alkyphenols. Applications and safety DNOC is an uncoupler, which means that it interferes with adenosine triphosphate (ATP) production, making it extremely toxic to humans. DNOC was one of the earliest pesticides developed, being used as an insecticide since the 1890s and a herbicide since the 1930s. It was banned for use as a pesticide in the United States in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrophenol
Nitrophenols are compounds of the formula HOC6H5−x(NO2)x. The conjugate bases are called nitrophenolates. Nitrophenols are more acidic than phenol itself. Mono-nitrophenols with the formula HOC6H4NO2. Three isomeric nitrophenols exist: * ''o''-Nitrophenol (2-nitrophenol; OH and NO2 groups are neighboring, a yellow solid. * ''m''-Nitrophenol (3-nitrophenol, CAS number: 554-84-7), a yellow solid (m.p. 97 °C) and precursor to the drug mesalazine (5-aminosalicylic acid). It can be prepared by nitration of aniline followed by replacement of the amino group via its diazonium derivative. * ''p''-Nitrophenol, yellow solid is a precursor to the rice herbicide fluorodifen, the pesticide parathion, and the human analgesic paracetamol (also known as acetaminophen). The mononitrated phenols are often hydrogenated to the corresponding aminophenols that are also useful industrially. Di- and trinitrophenols * 2,4-Dinitrophenol (m.p. 83 °C) is a moderately strong acid (pK ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution or more often as one of its salts such as hydroxylammonium sulfate, a water-soluble solid. Hydroxylamine and its salts are consumed almost exclusively to produce Nylon-6. The oxidation of to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Lobry de Bruyn and by the French chemist Léon Maurice Crismer (1858-1944). The coordination complex (zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone Methide
A quinone methide is a type of conjugated organic compound that contain a cyclohexadiene with a carbonyl and an exocyclic methylidene or extended alkene unit. It is analogous to a quinone, but having one of the double bonded oxygens replaced with a carbon. The carbonyl and methylidene are usually oriented either ortho or para to each other. There are some examples of transient synthetic meta quinone methides. Properties Quinone methides are cross-conjugated rather than aromatic. Nucleophilic addition at the exo-cyclic double bond will result in rearomatisation, making such reactions highly favourable. As a result, quinone methides are excellent, electrophilic Michael acceptors, react quickly with nucleophiles and can be easily reduced. They are able to act as radical scavengers via a similar process, a behaviour exploited by certain polymerisation inhibitors. Quinone methides are more polar than quinones, and therefore more chemically reactive. Simple unhindered quinone methid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoxyl Group
Aminoxyl denotes a radical functional group with general structure R2N–O•. It is commonly known as a nitroxyl radical or a nitroxide, however IUPAC discourages the use of these terms, as they erroneously suggest the presence of a nitro group. Aminoxyls are structurally related to hydroxylamines and ''N''-oxoammonium salts, with which they can interconvert via a series of redox steps. Aminoxyl radical.svg, The general structure of the aminoxyl radical 2,2,6,6-Tetramethylpiperidinyloxyl.svg, TEMPO, a commonly encountered organic aminoxyl radical Kaliumnitrosodisulfonat.svg, Fremy's salt, an inorganic aminoxyl radical Sterically unhindered aminoxyls bearing α-hydrogens are unstable and undergo rapid disproportionation to nitrones and hydroxylamines. Sterically hindered aminoxyls without α-hydrogens, such TEMPO and TEMPOL, and are persistent (stable) radicals and find use in a range of applications, both on the laboratory scale and in industry. Their ability to reversibly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylhydroxylamine
Diethylhydroxylamine (DEHA) is an organic compound with the formula (C2H5)2NOH. Strictly, this is ''N'',''N''-diethylhydroxylamine. It has an isomer, ''N'',''O''-diethylhydroxylamine with the formula EtNHOEt. Pure ''N'',''N''-diethylhydroxylamine is a colorless liquid, although it is usually encountered as a colourless-to-yellow solution in water with an amine-like odor. DEHA can be synthesised from a reaction between triethylamine and a peroxide. Applications DEHA is largely used as an oxygen scavenger in water treatment. It is a volatile oxygen scavenger and reacts in a ratio of 2.8/1 DEHA/O2. It is employed in high pressure (>70 bar) boiler systems due to a very low rate of reaction at low temperatures and pressures. Due to its volatility, it acts as an oxygen scavenger throughout the entire boiler system due to steam carryover. DEHA also reacts with ferrous metals to form a passivized film of magnetite throughout the boiler system. The reduction of toxic heavy metal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamines
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution or more often as one of its salts such as hydroxylammonium sulfate, a water-soluble solid. Hydroxylamine and its salts are consumed almost exclusively to produce Nylon-6. The Redox, oxidation of Ammonia, to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Lossen, Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Cornelis Adriaan Lobry van Troostenburg de Bruyn, Lobry de Bruyn and by the French ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenothiazine
Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history. The derivatives chlorpromazine and promethazine revolutionized the fields of psychiatry and allergy treatment, respectively. An earlier derivative, methylene blue, was one of the first antimalarial drugs, and derivatives of phenothiazine are currently under investigation as possible anti-infective drugs. Phenothiazine is a prototypical pharmaceutical lead structure in medicinal chemistry. Uses Phenothiazine itself is only of theoretical interest, but derivatives of it revolutionized psychiatry, other fields of medicine, and pest management. Other derivatives have been studied for possible use in advanced batteries and fuel cells. Phenothiazine-derived drugs In 1876, methylene blue, a derivative of phenothiazine, was synthesized by H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |