|
Polyelectrolyte Theory Of The Gene
The polyelectrolyte theory of the gene proposes that for a linear genetic biopolymer dissolved in water, such as DNA, to undergo Evolution, Darwinian evolution anywhere in the universe, it must be a polyelectrolyte, a polymer containing repeating Ionic charge, ionic charges. These charges maintain the uniform physical properties needed for Darwinian evolution, regardless of the information encoded in the Genetics, genetic biopolymer. DNA is such a molecule. Regardless of its nucleic acid sequence, the negative charges on its backbone dominate the physical interactions of the molecule to such a degree that it maintains uniform physical properties such as its Aqueous solution, aqueous solubility and double-helix structure. The polyelectrolyte theory of the gene was proposed by Steven A. Benner and Daniel Hutter in 2002 and has largely remained a theoretical framework astrobiologists have used to think about how life may be detected beyond Earth. This idea was later linked by Benner to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers (polymers of isoprene), suberin and lignin (complex polyphenolic polymers), cutin and cutan (complex polymers of long-chain fatty acids), melanin, and polyhydroxyalkanoates (PHAs). In addition to their many essential roles in living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatically
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges. Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), meaning 'amber', was thus the root of the word ''electricity''. Electrostatic phenomena arise from the forces that electric charges exert on each other. Such forces are described by Coulomb's law. There are many examples of electrostatic phenomena, from those as simple as the attraction of plastic wrap to one's hand after it is removed from a package, to the apparently spontaneous explosion of grain silos, the damage of electronic components during manufacturing, and photocopier and laser printer operation. The electrostatic model accurately predicts electrical phenomena in "classical" cases where the velocities are low and the system is macroscopic so no quantum effects are involved. It also plays a role in quantum mechanics, where additional t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
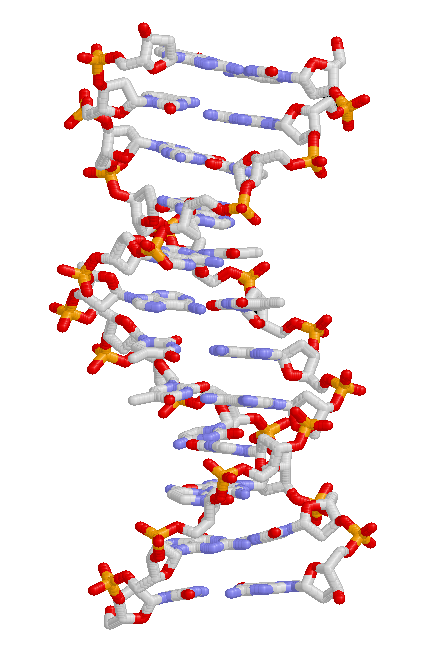
DNA Visual Representation Of Double-Helix
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of all known organisms and many viruses. DNA and ribonucleic acid (RNA) are nucleic acids. Alongside proteins, lipids and complex carbohydrates (polysaccharides), nucleic acids are one of the four major types of macromolecules that are essential for all known forms of life. The two DNA strands are known as polynucleotides as they are composed of simpler monomeric units called nucleotides. Each nucleotide is composed of one of four nitrogen-containing nucleobases (cytosine guanine adenine or thymine , a sugar called deoxyribose, and a phosphate group. The nucleotides are joined to one another in a chain by covalent bonds (known as the phosphodiester linkage) between the sugar of one nucleotide and the phosphate of the next, resulting in an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Guanine
Guanine () (symbol G or Gua) is one of the four main nucleotide bases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine ( uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is called guanosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine- imidazole ring system with conjugated double bonds. This unsaturated arrangement means the bicyclic molecule is planar. Properties Guanine, along with adenine and cytosine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA, and uracil only in RNA. Guanine has multiple tautomeric forms. For the imidazole ring, the proton can reside on either nitrogen. For the pyrimidine ring, the ring N-H can center can reside on either of the ring nitrogens. The latter tautomer does not apply to nucleoside or nucleotide versions of guanine. It binds to cytosine through three hydrogen bonds. In cytosine, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. Formation and reactions Structure Cystine is the disulfide derived from the amino acid cysteine. The conversion can be viewed as an oxidation: : Cystine contains a disulfide bond, two amine groups, and two carboxylic acid groups. As for other amino acids, the amine and carboxylic acid groups exist in rapid equilibrium with the ammonium-carboxylate tautomer. The great majority of the literature concerns the ''l,l-''cystine, derived from ''l''-cysteine. Other isomers include ''d,d''-cystine and the meso isomer d,l-cystine, neither of which is biologically significant. Occurrence Cystine is common in many foods such as eggs, meat, dairy products, and whole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymine
Thymine () (symbol T or Thy) is one of the four nucleotide bases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. In RNA, thymine is replaced by the nucleobase uracil. Thymine was first isolated in 1893 by Albrecht Kossel and Albert Neumann from calf thymus glands, hence its name. Derivation As its alternate name (5-methyluracil) suggests, thymine may be derived by methylation of uracil at the 5th carbon. In RNA, thymine is replaced with uracil in most cases. In DNA, thymine (T) binds to adenine (A) via two hydrogen bonds, thereby stabilizing the nucleic acid structures. Thymine combined with deoxyribose creates the nucleoside deoxythymidine, which is synonymous with the term thymidine. Thymidine can be phosphorylated with up to three phosphoric acid groups, producing dTMP (deoxythymidine monophosphate), dTDP, or dTTP (for the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenine
Adenine (, ) (nucleoside#List of nucleosides and corresponding nucleobases, symbol A or Ade) is a purine nucleotide base that is found in DNA, RNA, and Adenosine triphosphate, ATP. Usually a white crystalline subtance. The shape of adenine is complementary and pairs to either thymine in DNA or uracil in RNA. In cells adenine, as an independent molecule, is rare. It is almost always covalent bond, covalently bound to become a part of a larger biomolecule. Adenine has a central role in cellular respiration. It is part of adenosine triphosphate which provides the energy that drives and supports most activities in living cell (biology), cells, such as Protein biosynthesis, protein synthesis, chemical synthesis, muscle contraction, and nerve impulse propagation. In respiration it also participates as part of the cofactor (biochemistry), cofactors nicotinamide adenine dinucleotide, flavin adenine dinucleotide, and Coenzyme A. It is also part of adenosine, adenosine monophosphate, cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleobase
Nucleotide bases (also nucleobases, nitrogenous bases) are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic building blocks of nucleic acids. The ability of nucleobases to form base pairs and to stack one upon another leads directly to long-chain helical structures such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Five nucleobases— adenine (A), cytosine (C), guanine (G), thymine (T), and uracil (U)—are called ''primary'' or ''canonical''. They function as the fundamental units of the genetic code, with the bases A, G, C, and T being found in DNA while A, G, C, and U are found in RNA. Thymine and uracil are distinguished by merely the presence or absence of a methyl group on the fifth carbon (C5) of these heterocyclic six-membered rings. In addition, some viruses have aminoadenine (Z) instead of adenine. It differs in having an extra amine group, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
What Is Life?
''What Is Life? The Physical Aspect of the Living Cell'' is a 1944 science book written for the lay reader by the physicist Erwin Schrödinger. The book was based on a course of public lectures delivered by Schrödinger in February1943, under the auspices of the Dublin Institute for Advanced Studies, where he was Director of Theoretical Physics, at Trinity College, Dublin. The lectures attracted an audience of about 400, who were warned "that the subject-matter was a difficult one and that the lectures could not be termed popular, even though the physicist’s most dreaded weapon, mathematical deduction, would hardly be utilized." Margulis, Lynn. & Sagan, Dorion. (1995). ''What Is Life?'' (pg. 1). Berkeley: University of California Press. Schrödinger's lecture focused on one important question: "how can the events in space and time which take place within the spatial boundary of a living organism be accounted for by physics and chemistry?" In the book, Schrödinger introduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Descent With Modification
Evolution is the change in the heritable Phenotypic trait, characteristics of biological populations over successive generations. It occurs when evolutionary processes such as natural selection and genetic drift act on genetic variation, resulting in certain characteristics becoming more or less common within a population over successive generations. The process of evolution has given rise to biodiversity at every level of biological organisation. The scientific theory of evolution by natural selection was conceived independently by two British naturalists, Charles Darwin and Alfred Russel Wallace, in the mid-19th century as an explanation for why organisms are adapted to their physical and biological environments. The theory was first set out in detail in Darwin's book ''On the Origin of Species''. Evolution by natural selection is established by observable facts about living organisms: (1) more offspring are often produced than can possibly survive; (2) phenotypic variatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphodiester Bond
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is dominated by their prevalence in DNA and RNA, but phosphodiesters occur in other biomolecules, e.g. acyl carrier proteins, Phospholipid, phospholipids and the cyclic forms of GMP and AMP (Cyclic guanosine monophosphate, cGMP and Cyclic adenosine monophosphate, cAMP). Phosphodiester Backbone of DNA and RNA Phosphodiester bonds make up the Polymer backbone, backbones of DNA and RNA. In the phosphodiester bonds of nucleic acids, a phosphate is attached to the 5' carbon of one nucleoside and to the 3' carbon of the adjacent nucleoside. Specifically, it is the phosphodiester bonds that link the Directionality (molecular biology), 3' carbon atom of one sugar molecule and the 5' carbon atom of another (hence the name 3', 5' phosphodiester linkag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, phosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one proton gives the dihydrogen phosphate ion while removal of two protons gives the hydrogen phosphate ion . These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate or orthophosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |