|
Pirenperone Synthesis
Pirenperone (, , ; developmental code names R-47456, R-50656) is a serotonin receptor antagonist described as an antipsychotic and tranquilizer which was never marketed. It is a relatively binding selectivity, selective receptor antagonist, antagonist of the serotonin receptor, serotonin 5-HT2 receptor, 5-HT2 receptors and has been used in scientific research to study the serotonin system. In the 1980s, the drug was found to block the effects of the lysergic acid diethylamide (LSD) in animals, and along with ketanserin, led to the elucidation of the 5-HT2A receptor, 5-HT2A receptor as the biological target, biological mediator of the effects of serotonergic psychedelics. Synthesis The sidechain is formed from the cyclization of 2-aminopyridine with 2-Acetylbutyrolactone (2) in PPA gave 3-(2-hydroxyethyl)-2-methylpyrido[1,2-a]pyrimidin-4-one [41078-67-5] (3). Halogenation replaces the hydroxy with the chloride leaving group. Many syntheses do both steps in one-pot with phosphorus o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin Receptor Antagonist
A serotonin antagonist, or serotonin receptor antagonist, is a drug used to inhibit the action at serotonin (5-HT) receptors. Types 5-HT2A antagonists Antagonists of the 5-HT2A receptor are sometimes used as atypical antipsychotics (contrast with typical antipsychotics, which are purely dopamine antagonists). They include, but are not limited to: * Cyproheptadine blocks 5-HT2A, H1 and is a mild anticholinergic. * Methysergide is a 5-HT2A antagonist and nonselective 5-HT1 receptor blocker. It causes retroperitoneal fibrosis and mediastinal fibrosis. * Quetiapine blocks 5-HT2A, 5-HT1A, dopamine receptors D1 and D2, histamine receptor H1, and A1 adrenoreceptors. 5-HT2A/2C antagonists * Ketanserin Antihypertensive. Blocks 5-HT2A, 5-HT2C and Alpha 1 (A1) adrenoreceptors. * Risperidone antipsychotic * Trazodone 5-HT3 antagonists Another subclass consists of drugs selectively acting at the 5-HT3 receptors, and thus are known as 5-HT3 antagonists. They are efficacious ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate. Structure Like phosphate, is tetrahedral in shape. It features three P−Cl bonds and one strong P=O double bond, with an estimated bond dissociation energy of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is dominant, in contrast with the case of . The P=O bond involves the donation of the lone pair electrons on oxygen ''p''-orbitals to the antibonding combinations associated with phosphorus-chlorine bonds, thus constituting ''π'' bonding. Phosphoryl chloride exists as neutral molecules in the solid, liquid and gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anxiolytics
An anxiolytic (; also antipanic or antianxiety agent) is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms. Nature of anxiety Anxiety is a naturally-occurring emotion and an innate response of the body to the environmental stimuli. Mild to moderate anxiety would increase level of performance. However, when anxiety levels exceed the tolerability of a person, anxiety disorders may occur. People with anxiety disorders can exhibit fear responses such as defensive behaviors, high levels of alertness and negative emotions, without external stimuli which induce anxiety within an individual. Those with anxiety disorders are also often found to have concurrent psychological disorders, most commonly depression. Anxiety disorders are divided into 6 types in clinical recognition. They are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antipsychotics
Antipsychotics, also known as neuroleptics, are a class of psychotropic medication primarily used to manage psychosis (including delusions, hallucinations, paranoia or disordered thought), principally in schizophrenia but also in a range of other psychotic disorders. They are also the mainstay together with mood stabilizers in the treatment of bipolar disorder. Prior research has shown that use of any antipsychotic is associated with smaller brain tissue volumes, including white matter reduction and that this brain shrinkage is dose dependent and time dependent. A more recent controlled trial suggests that second generation antipsychotics combined with intensive psychosocial therapy may potentially prevent pallidal brain volume loss in first episode psychosis. The use of antipsychotics may result in many unwanted side effects such as involuntary movement disorders, gynecomastia, impotence, weight gain and metabolic syndrome. Long-term use can produce adverse effects su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-HT2 Antagonists
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ocaperidone
Ocaperidone (R 79598) is a benzisoxazole antipsychotic. It was initially developed by Janssen, later licensed to French laboratory Neuro3D and then acquired in 2007 by German company Evotec. It was found to be more potent than risperidone in animal studies, but its testing was abandoned in 2010 after unfavorable results in human Phase II trials, as while it was effective at controlling symptoms of schizophrenia, ocaperidone produced an unacceptable amount of extrapyramidal side effects.Geerts H, Spiros A, Roberts P, Twyman R, Alphs L, Grace AA. Blinded prospective evaluation of computer-based mechanistic schizophrenia disease model for predicting drug response. ''PLoS One''. 2012;7(12):e49732. Synthesis The last step requires attachment of the sidechain between 3-(2-bromoethyl)-2,9-dimethyl 4H-pyrido,2-ayrimidin-4-oneCID:18995805(1) and 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole 4163-77-9(2) completing the convergent synthesis of Ocaperidone (3).. See also * Benperidol * T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
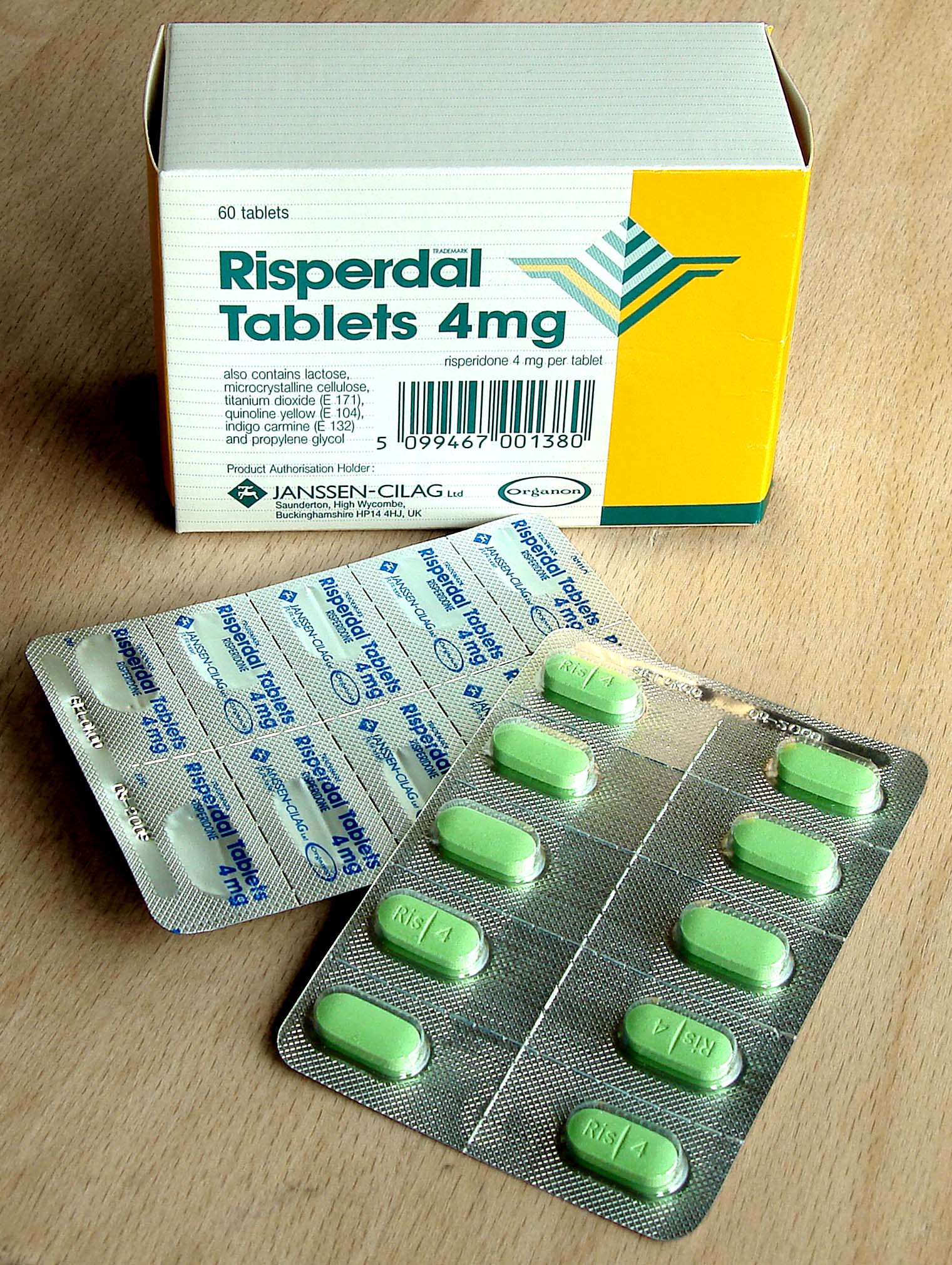
Risperidone
Risperidone, sold under the brand name Risperdal among others, is an atypical antipsychotic used to treat schizophrenia and bipolar disorder. It is taken either by mouth or by injection (subcutaneous or intramuscular). The injectable versions are long-acting and last for 2–4 weeks. Common side effects include movement problems, sleepiness, dizziness, trouble seeing, constipation, and increased weight. Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, as well as neuroleptic malignant syndrome, an increased risk of suicide, and high blood sugar levels. In older people with psychosis as a result of dementia, it may increase the risk of death. It is unknown if it is safe for use in pregnancy. Its mechanism of action is not entirely clear, but is believed to be related to its action as a dopamine and serotonin antagonist. Study of risperidone began in the late 1980s and it was approved for sale in the United States in 1993. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Setoperone
Setoperone is a compound that is a ligand to the 5-HT2A receptor. It can be radiolabeled with the radioisotope fluorine-18 and used as a radioligand with positron emission tomography (PET). Several research studies have used the radiolabeled setoperone in neuroimaging for the studying neuropsychiatric disorders, such as depression or schizophrenia. Synthesis The starting material is called 6-(2-hydroxyethyl)-7-methyl-2,3-dihydro- ,3hiazolo,2-ayrimidin-5-oneCID:15586462(1). Halogenation of this with hydrobromic acid in acetic acid giveCID:15586463(2). Sn2 alkylation with 4-(4-fluorobenzoyl)piperidine 6346-57-7(3) under Finkelstein reaction conditions affords setoperone (4). See also * Altanserin * Ketanserin * Pirenperone * Ritanserin Ritanserin, also known by its developmental code name R-55667, is a serotonin antagonist medication described as an anxiolytic, antidepressant, antiparkinsonian agent, and antihypertensive agent. It was never marketed for medical use due ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketanserin
Ketanserin (INN, USAN, BAN) (brand name Sufrexal; former developmental code name R41468) is a drug used clinically as an antihypertensive agent and in scientific research to study the serotonin system; specifically, the 5-HT2 receptor family. It was discovered at Janssen Pharmaceutica in 1980. It is not available in the United States. Uses Medical uses Ketanserin is classified as an antihypertensive by the World Health Organization and the National Institute of Health. It has been used to reverse pulmonary hypertension caused by protamine (which in turn was administered to reverse the effects of heparin overdose). The reduction in hypertension is not associated with reflex tachycardia. It has been used in cardiac surgery. A 2000 Cochrane Review found that, compared to placebo, ketanserin did not provide significant relief for people suffering from Raynaud's phenomenon attacks in the setting of progressive systemic sclerosis (an autoimmune disorder). While the frequency of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Altanserin
Altanserin is a compound that binds to the 5-HT2A receptor (5-Hydroxytryptamine (serotonin) 2A receptor). Labeled with the isotope fluorine-18 it is used as a radioligand in positron emission tomography (PET) studies of the brain, i.e., studies of the 5-HT2A neuroreceptors. Besides human neuroimaging studies altanserin has also been used in the study of rats. An alternative for PET imaging the 5-HT2A receptor is the [ 11C] volinanserin (MDL-100,907) radioligand. 18F-altanserin and 3H-volinanserin have shown very comparable binding. Both altanserin and MDL 100,907 are 5-HT2A receptor antagonists. 18F">sup>18F setoperone can also be used in PET. An alternative SPECT radioligand is the 123I">sup>123I5-I-R91150 receptor antagonist. A rapid chemical synthesis of fluorine-18 and H-2 dual-labeled altanserin has been described. Other ligands for other parts of the serotonin system used in PET studies are, e.g., DASB, ketanserin, and WAY-100635. Human brain map ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |