|
Phenazone
Phenazone (International Nonproprietary Name, INN and British Approved Name, BAN; also known as phenazon, antipyrine (United States Adopted Name, USAN), antipyrin, or analgesine) is an analgesic (pain reducing), antipyretic (fever reducing) and anti-inflammatory drug. While it predates the term, it is often classified as a nonsteroidal anti-inflammatory drug (NSAID). Phenazone was one of the earliest synthetic medications — when it was patented in 1883, the only synthetic medical chemicals on the market were chloral hydrate, a sedative (as well as at least one derivative of that chemical), trimethylamine, and iodol (tetraiodopyrrol), an early antiseptic. One of the earliest widely used analgesics and antipyretics, phenazone was gradually replaced in common use by other medications including phenacetin (itself later withdrawn because of safety concerns), aspirin, paracetamol and modern NSAIDs such as ibuprofen. However, it is still available in several countries either as an over-t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazolone
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional ketone, carbonyl (C=O) group. Compounds containing this functional group are useful commercially in Analgesic, analgesics and dyes. Structure and synthesis Pyrazolone can exist in two isomers: 3-pyrazolone and 4-pyrazolone. These isomers can interconvert via lactam–lactim and imine–enamine tautomerism; these conversion often display photochromism. For pyrazolone derivatives, the 3-pyrazolone isomer can be stabilized with ''N''-alkyl or ''N''-aryl substituents. : The first synthesis of pyrazolones was reported in 1883 by Ludwig Knorr, via a condensation reaction between ethyl acetoacetate and phenylhydrazine. : Many pyrazolones are produced by functionalization of preformed pyrazolones. Applications Pharmaceuticals Pyrazolones are amongst the oldest synthetic pharmaceuticals, starting with the introduction of antipyrine (ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ludwig Knorr
Ludwig Knorr (2 December 1859 – 4 June 1921) was a German chemist. Together with Carl Paal, he discovered the Paal–Knorr synthesis, and the Knorr quinoline synthesis and Knorr pyrrole synthesis are also named after him. The synthesis in 1883 of the analgesic drug antipyrine, now called phenazone, was a commercial success. Antipyrine was the first synthetic drug and the most widely used drug until it was replaced by Aspirin in the early 20th century. Early life Ludwig Knorr was born to a wealthy merchant family in 1859. He grew up in the Sabbadini-Knorr company headquarters, located in the Kaufingerstraße in the center of Munich, and in the family house near the Lake Starnberg. After the early death of his father, the education of him and his four brothers lay in the hands of their mother. In 1878 Knorr received his Abitur and started to study chemistry at the University of Munich. In the beginning, he studied under Jacob Volhard; then, after Volhard left for the University ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic, antalgic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, recent research has suggested that classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants may be considered as an alternative. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing to the substantial risks and high chances of overdose, misuse, and addiction in the absence of medical supervision. Etymology The word ''analgesic'' derive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agranulocytosis
Agranulocytosis, also known as agranulosis or granulopenia, is an acute condition involving a severe and dangerous lowered white blood cell count (leukopenia, most commonly of neutrophils) and thus causing neutropenia in the circulating blood. It is a severe lack of one major class of infection-fighting white blood cells. People with this condition are at very high risk of serious infections due to their suppressed immune system. In agranulocytosis, the concentration of granulocytes (a major class of white blood cells that includes neutrophils, basophils, and eosinophils) drops below 200 cells/mm3 of blood. Signs and symptoms Agranulocytosis may be asymptomatic, or may clinically present with sudden fever, rigors and sore throat. Infection of any organ may be rapidly progressive (e.g., pneumonia, urinary tract infection). Sepsis may also progress rapidly. Causes A large number of drugs have been associated with agranulocytosis, including antiepileptics (such as carbamaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenacetin
Phenacetin (; acetophenetidin, ''N''-(4-ethoxyphenyl)acetamide) is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s (e.g., withdrawn in Canada in 1973, and by the U.S. Food and Drug Administration in 1983). History Phenacetin was introduced in 1887 in Elberfeld, Germany by German company Bayer, and was used principally as an analgesic; it was one of the first synthetic fever reducers to go on the market. It is also known historically to be one of the first non- opioid analgesics without anti-inflammatory properties. Although paracetamol (acetaminophen) was produced earlier, a historical accident saw it ignored after Joseph von Mering's 1893 assessment. Prior to World War One, Britain imported phenacetin from Germany. During the war, a team including Jocelyn Field Thorpe and Martha Annie Whiteley developed a synthesis in Britain. Known mechanism of action Phen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. Preparation At large scale, ethyl acetoacetate is industrially produced by treatment of diketene with ethanol. The small scale preparation of ethyl acetoacetate is a classic laboratory procedure. It involves Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol. : Reactions Ethyl acetoacetate is subject to keto-enol tautomerism. In the neat liquid at 33 °C, the enol consists of 8% of the total. The enol is moderately acidic. Thus ethyl acetoacetate behaves similarly to acetylacetone: : The resulting carbanion undergoes nucleophilic substitution. Ethyl acetoacetate is often used in the acetoacetic ester synthesis, comparable to diethyl malonate in the malonic ester synthesis or the Kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzocaine
Benzocaine, sold under the brand name Orajel amongst others, is a local anesthetic, belonging to the amino ester drug class, commonly used as a topical painkiller or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments such as products for oral ulcers. It is combined with antipyrine to form A/B ear drops. In the US, products containing benzocaine for oral application are contraindicated in children younger than two years old. In the European Union, the contraindication applies to children under 12 years of age. It was first synthesised in 1890 in Germany and approved for medical use in 1902. Medical uses Benzocaine is indicated to treat a variety of pain-related conditions. It may be used for: * Local anesthesia of oral and pharyngeal mucous membranes (sore throat, cold sores, mouth ulcers, toothache, sore gums, denture irritation)AHFS Drug Information 2007. McEvoy GK, ed. Benzocaine. Bethesda, MD: American Society of Health- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A/B Otic Drops
Antipyrine and benzocaine ear drops is a medication for the treatment of ear pain caused by otitis media. It combines antipyrine, an NSAID, and benzocaine, a local anaesthetic in order to treat ear pain, alongside hydroxyquinoline sulfate, an antiseptic and preservative. Its trade names include Auralgan, Aurodex, Auroto, among others, and is abbreviated as A/B otic drops. Medical uses A/B otic drops is indicated for ear pain caused by otitis media. It is used every 2–3 hours as needed for pain. A/B otic drops is also indicated for the removal of excessive or impacted cerumen. To clear cerumen, it is used 3 times a day for 2–3 days. Ingredients Each 1 ml of A/B otic drops contains: * Antipyrine 54 mg * Benzocaine 14 mg * Glycerin and Hydroxyquinoline Sulfate USP Clinical pharmacology A/B otic drops are effective because antipyrine reduces pain and inflammation and benzocaine numbs the ear. Reformulation In 2008, Auralgan was reformulated to include ace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatotoxicity
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury (DILI) is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. acetaminophen, paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., alpha-amanitin), and herbal remedies (two prominent examples being kava, though the causal mechanism is unknown, and comfrey, through pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. It can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the throat. Over 30 definitions of nausea were proposed in a 2011 book on the topic. Nausea is a non-specific symptom, which means that it has many possible causes. Some common causes of nausea are gastroenteritis and other gastrointestinal disorders, food poisoning, motion sickness, dizziness, migraine, fainting, low blood sugar, anxiety, hyperthermia, dehydration and lack of sleep. Nausea is a side effect of many medications including chemotherapy, or morning sickness in early pregnancy. Nausea may also be caused by disgust and depression. Medications taken to prevent and treat nausea and vomiting are called antiemetics. The most commonly prescribed antiemetics in the US are promethazine, metoclopramide, and the newer ondansetron. The word na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
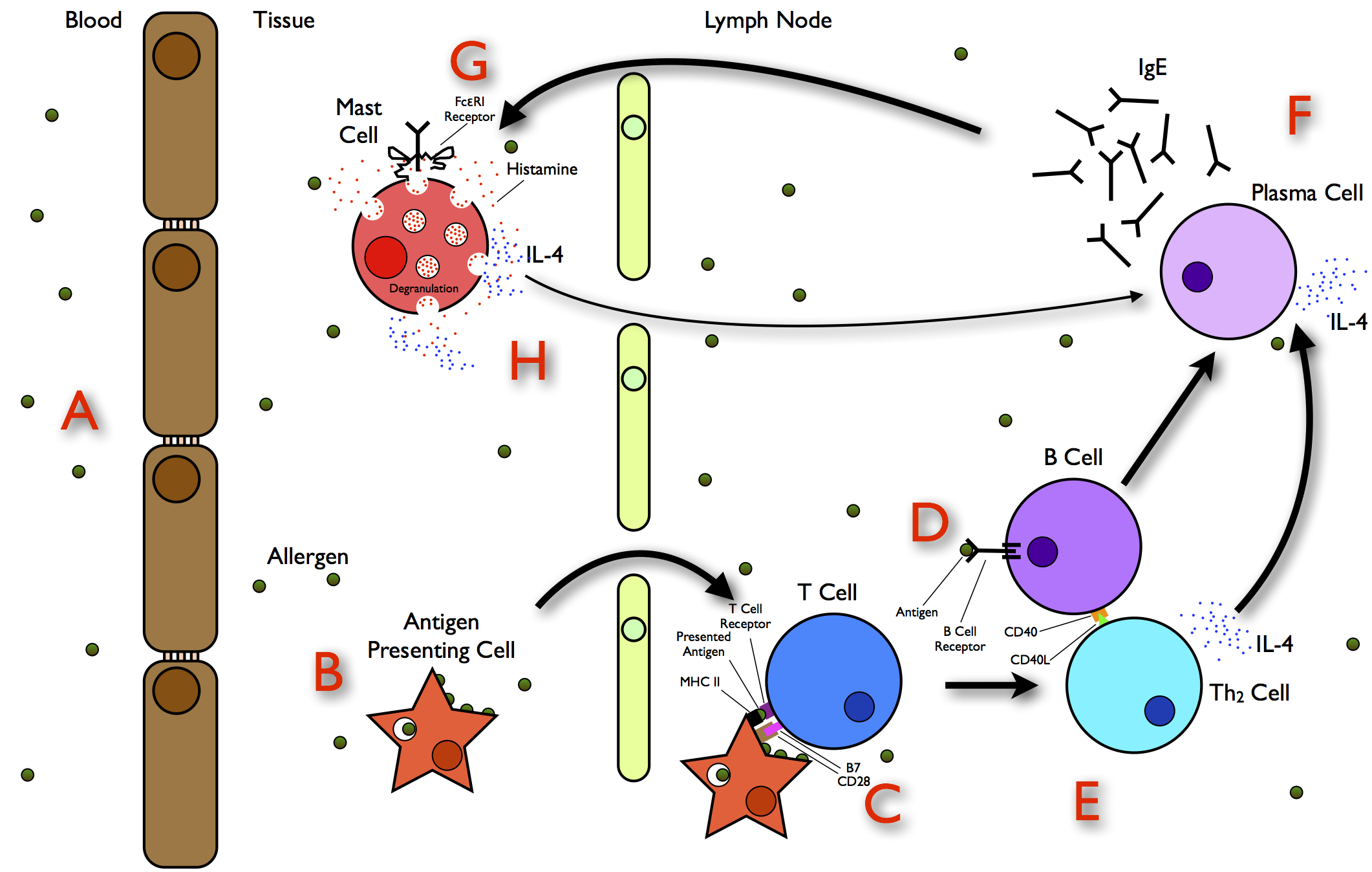
Allergy
Allergies, also known as allergic diseases, are various conditions caused by hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include Allergic rhinitis, hay fever, Food allergy, food allergies, atopic dermatitis, allergic asthma, and anaphylaxis. Symptoms may include allergic conjunctivitis, red eyes, an itchy rash, sneeze, sneezing, coughing, a rhinorrhea, runny nose, shortness of breath, or swelling. Note that food intolerances and food poisoning are separate conditions. Common allergens include pollen and certain foods. Metals and other substances may also cause such problems. Food, insect stings, and medications are common causes of severe reactions. Their development is due to both genetic and environmental factors. The underlying mechanism involves immunoglobulin E antibodies (IgE), part of the body's immune system, binding to an allergen and then to FcεRI, a receptor on mast cells or basophils where it triggers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidize
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |