|
Penicillin Binding Proteins
Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics (except for tabtoxinine-β-lactam, which inhibits glutamine synthetase) bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of transpeptidase enzymes called DD-transpeptidases. Diversity There are a large number of PBPs, usually several in each organism, and they are found as both membrane-bound and cytoplasmic proteins. For example, Spratt (1977) reports that six different PBPs are routinely detected in all strains of '' E. coli'' ranging in molecular weight from 40,000 to 91,000. The different PBPs occur in different numbers per cell and have varied affinities for penicillin. The PBPs are usually broadly classified into high-molecular-weight (HMW) and low ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently it is classified as a non-polar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as ) and its carboxyl group deprotonated (as ). It is non-essential to humans as it can be synthesized metabolically and does not need to be present in the diet. It is encoded by all codons starting with G C (GC U, GCC, GC A, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to L-leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in peptides in some bacterial cell walls (in peptidoglycan) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filamentation
Filamentation is the anomalous growth of certain bacteria, such as ''Escherichia coli'', in which cells continue to elongate but do not divide (no septa formation). The cells that result from elongation without division have multiple chromosomal copies. In the absence of antibiotics or other stressors, filamentation occurs at a low frequency in bacterial populations (4–8% short filaments and 0–5% long filaments in 1- to 8-hour cultures). The increased cell length can protect bacteria from protozoan predation and neutrophil phagocytosis by making ingestion of cells more difficult. Filamentation is also thought to protect bacteria from antibiotics, and is associated with other aspects of bacterial virulence such as biofilm formation. The number and length of filaments within a bacterial population increases when the bacteria are exposed to different physical, chemical and biological agents (e.g. UV light, DNA synthesis-inhibiting antibiotics, bacteriophages). This is terme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenicillin
Carbenicillin is a bactericidal antibiotic belonging to the carboxypenicillin subgroup of the penicillins. It was discovered by scientists at Beecham and marketed as Pyopen. It has Gram-negative coverage which includes ''Pseudomonas aeruginosa'' but limited Gram-positive coverage. The carboxypenicillins are susceptible to degradation by beta-lactamase enzymes, although they are more resistant than ampicillin to degradation. Carbenicillin is also more stable at lower pH than ampicillin. Pharmacology The antibiotic is highly soluble in water and is acid-labile. A typical lab working concentration is 50 to 100 μg per mL. It is a semi-synthetic analogue of the naturally occurring benzylpenicillin. Carbenicillin at high doses can cause bleeding. Use of carbenicillin can cause hypokalemia by promoting potassium loss at the distal convoluted tubule of the kidney. In molecular biology, carbenicillin may be preferred as a selecting agent (see plasmid stabilisation technology) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Site Of PBP3 With Carbenicillin
Active may refer to: Music * ''Active'' (album), a 1992 album by Casiopea * "Active" (song), a 2024 song by Asake and Travis Scott from Asake's album ''Lungu Boy'' * Active Records, a record label Ships * ''Active'' (ship), several commercial ships by that name * HMS ''Active'', the name of various ships of the British Royal Navy * USCS ''Active'', a US Coast Survey ship in commission from 1852 to 1861 * USCGC ''Active'', the name of various ships of the US Coast Guard * USRC ''Active'', the name of various ships of the US Revenue Cutter Service * USS ''Active'', the name of various ships of the US Navy Computers and electronics * Active Enterprises, a defunct video game developer * Sky Active, the brand name for interactive features on Sky Digital available in the UK and Ireland * Active (software), software used for open publishing by Indymedia; see Independent Media Center * The "live" circuit of mains power in countries observing AS/NZS 3112 electrical standa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
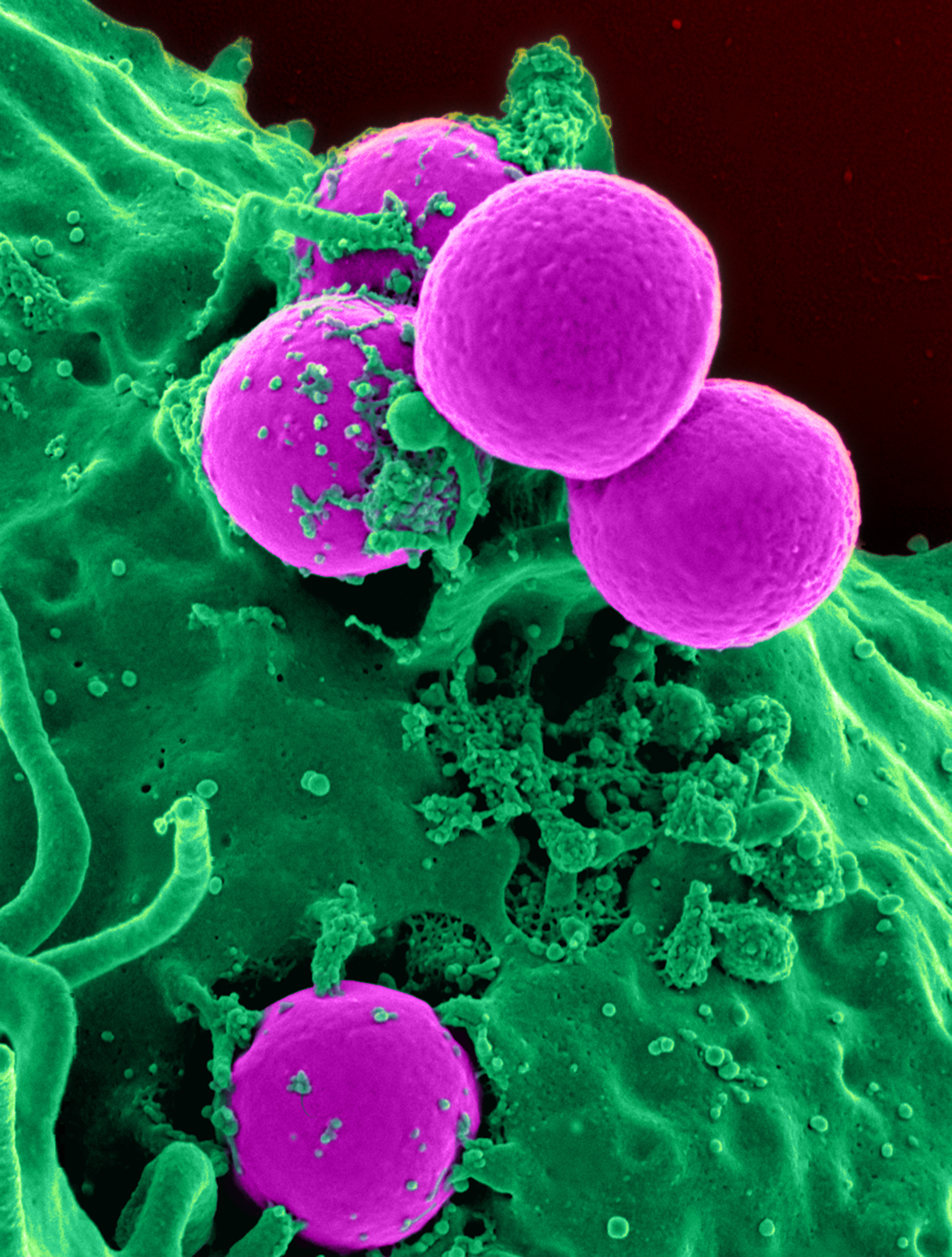
Methicillin-resistant Staphylococcus Aureus
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019. MRSA is any strain of ''S. aureus'' that has developed (through mutation) or acquired (through horizontal gene transfer) a multiple drug resistance to beta-lactam antibiotics. Beta-lactam (β-lactam) antibiotics are a broad-spectrum group that include some penams (penicillin derivatives such as methicillin and oxacillin) and cephems such as the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-susceptible ''S. aureus'', or MSSA. MRSA infection is common in hospitals, prisons, and nursing homes, where people with open wounds, invasive devices such as catheters, and weakened immune systems are at greate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Resistance
Antimicrobial resistance (AMR or AR) occurs when microbes evolve mechanisms that protect them from antimicrobials, which are drugs used to treat infections. This resistance affects all classes of microbes, including bacteria (antibiotic resistance), viruses (antiviral resistance), Parasitic disease, parasites (antiparasitic resistance), and fungi (antifungal resistance). Together, these adaptations fall under the AMR umbrella, posing significant challenges to healthcare worldwide. Misuse and improper management of antimicrobials are primary drivers of this resistance, though it can also occur naturally through genetic mutations and the spread of resistant genes. Antibiotic resistance, a significant AMR subset, enables bacteria to survive antibiotic treatment, complicating infection management and treatment options. Resistance arises through spontaneous mutation, horizontal gene transfer, and increased selective pressure from Antibiotic misuse, antibiotic overuse, both in medicin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Penicillin Binding Protein 2A
''mecA'' is a gene found in bacterial cells which allows them to be resistant to antibiotics such as methicillin, penicillin and other penicillin-like antibiotics. The bacteria strain most commonly known to carry ''mecA'' is methicillin-resistant ''Staphylococcus aureus'' (MRSA). In ''Staphylococcus'' species, ''mecA'' is spread through the staphylococcal chromosome cassette SCC''mec'' genetic element. Resistant strains cause many hospital-acquired infections. ''mecA'' encodes the protein PBP2A ( penicillin-binding protein 2A), a transpeptidase that helps form the bacterial cell wall. PBP2A has a lower affinity for beta-lactam antibiotics such as methicillin and penicillin than DD-transpeptidase does, so it does not bind to the ringlike structure of penicillin-like antibiotics. This enables transpeptidase activity in the presence of beta-lactams, preventing them from inhibiting cell wall synthesis. The bacteria can then replicate as normal. History Methicillin resistance fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactamase
Beta-lactamases (β-lactamases) are enzymes () produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactamases produced by gram-negative bacteria are usually secreted, especially when antibiotics are present in the environment. Structure The structure of a ''Streptomyces'' serine β-lactamase (SBLs) is given by . The alpha-beta fold () resembles that of a DD-transpeptidase, from which the enzyme is thought to have evolved. β-lactam antibiotics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MreB
MreB is a protein found in bacteria that has been identified as a homologue of actin, as indicated by similarities in tertiary structure and conservation of active site peptide sequence. The conservation of protein structure suggests the common ancestry of the cytoskeletal elements formed by actin, found in eukaryotes, and MreB, found in prokaryotes. Indeed, recent studies have found that MreB proteins polymerize to form filaments that are similar to actin microfilaments. It has been shown to form multilayer sheets comprising diagonally interwoven filaments in the presence of ATP or GTP. MreB along with MreC and MreD are named after the mre operon (murein formation gene cluster E) to which they all belong. Function MreB controls the width of rod-shaped bacteria, such as ''Escherichia coli''. A mutant ''E. coli'' that creates defective MreB proteins will be spherical instead of rod-like. Also, most bacteria that are naturally spherical do not have the gene encoding MreB. M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminal End
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |