|
Parahopeite
Zinc phosphate is an inorganic compound with the formula Zn3( PO4)2. This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment (see also red lead). It has largely displaced toxic materials based on lead or chromium, and by 2006 it had become the most commonly used corrosion inhibitor. Zinc phosphate coats better on a crystalline structure than bare metal, so a seeding agent is often used as a pre-treatment. One common agent is sodium pyrophosphate. Minerals Natural forms of zinc phosphate include minerals hopeite and parahopeite. A somewhat similar mineral is natural hydrous zinc phosphate called tarbuttite, Zn2(PO4)(OH). Both are known from oxidation zones of Zn ore beds and were formed through oxidation of sphalerite Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hopeite
Hopeite is a hydrated zinc phosphate with formula: Zn3(PO4)2·4H2O. It is a rare mineral used mainly as a collectors specimen. Hopeite crystallizes in the orthorhombic system with prismatic, vitreous white to yellow crystals. It also forms druzy encrustations and reniform (kidney-shaped) masses. The related mineral parahopeite, which has the same composition but different crystal structure, is triclinic. The minerals are formed through oxidation of sphalerite by the presence of phosphate-rich solutions It was first described in 1822 from Moresnet, Liège Province, Belgium and is named after Scottish Scottish usually refers to something of, from, or related to Scotland, including: *Scottish Gaelic, a Celtic Goidelic language of the Indo-European language family native to Scotland *Scottish English *Scottish national identity, the Scottish ide ... chemist, Thomas Charles Hope (1766–1844) of the University of Edinburgh. It has been found in Zambia associated with la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parahopeite
Zinc phosphate is an inorganic compound with the formula Zn3( PO4)2. This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment (see also red lead). It has largely displaced toxic materials based on lead or chromium, and by 2006 it had become the most commonly used corrosion inhibitor. Zinc phosphate coats better on a crystalline structure than bare metal, so a seeding agent is often used as a pre-treatment. One common agent is sodium pyrophosphate. Minerals Natural forms of zinc phosphate include minerals hopeite and parahopeite. A somewhat similar mineral is natural hydrous zinc phosphate called tarbuttite, Zn2(PO4)(OH). Both are known from oxidation zones of Zn ore beds and were formed through oxidation of sphalerite Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tarbuttite
Tarbuttite is a rare phosphate mineral with formula Zn2(PO4)(OH). It was discovered in 1907 in what is now Zambia and named for Percy Tarbutt. Description and habit Tarbuttite is white, yellow, red, green, brown, or colorless; in transmitted light it is colorless. Traces of copper cause green coloring, while iron hydroxides cause the other colors. Colorless crystals tend to be transparent while colored specimens have varying degrees of transparency. The mineral occurs as equant to short prismatic crystals up to , in sheaf-like or saddle-shaped aggregates, or as crusts. Individual crystals are commonly rounded and striated. Structure Zinc ions are surrounded by oxygen in a nearly perfect trigonal bipyramid and phosphate groups are tetrahedral. The crystal structure consists of zig-zag chains of Zn1 polyhedra linked by phosphate groups and pairs of Zn2 polyhedra. In each unit cell are two formula units of Zn2(PO4)(OH).Cocco, p. 321. History Tarbuttite was discovered in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temporary Restoration
Temporary restoration is a temporary filling of a prepared tooth until permanent restoration is carried out. It is used to cover the prepared part of the tooth, in order to maintain the occlusal space and the contact points, and insulation of the pulpal tissues and maintenance of the periodontal relationship. Sometimes permanent restoration is not done after tooth preparation; this may be to prepare for indirect restoration such as inlays and onlays. Temporary fillings are also used for 'stabilization' techniques where many restorations are needed, and the problem may become worse before it can be fully treated – so temporary fillings are placed in order to stop progression. Materials used *Zinc oxide eugenol Zinc oxide eugenol (ZOE) is a material created by the combination of zinc oxide and eugenol contained in oil of cloves. An acid-base reaction takes place with the formation of zinc eugenolate chelate. The reaction is catalysed by water and is acc ... *Intermediate Resto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
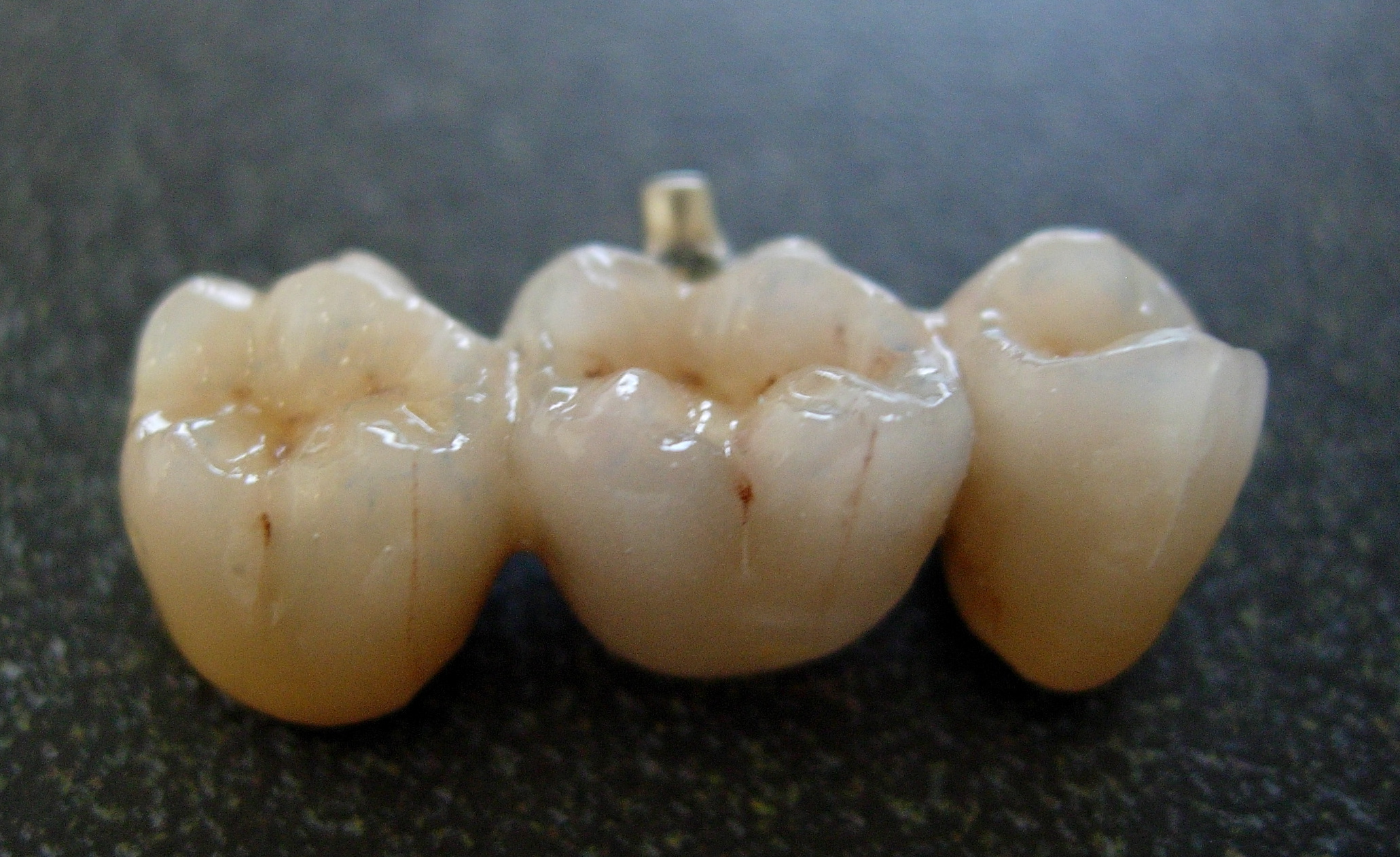
Crown (dentistry)
In dentistry, a crown most commonly refers to a dental cap, a type of dental restoration that completely caps or encircles a tooth or dental implant. A crown may be needed when a large cavity threatens the health of a tooth. A crown is typically bonded to the tooth by dental cement. They can be made from various materials, which are usually fabricated using ''indirect methods''. Crowns are used to improve the strength or appearance of teeth and to halt deterioration. While beneficial to dental health, the procedure and materials can be costly. The most common method of crowning a tooth involves taking a dental impression of a tooth prepared by a dentist, then fabricating the crown outside of the mouth. The crown can then be inserted at a subsequent dental appointment. This ''indirect method'' of tooth restoration allows use of strong restorative material requiring time-consuming fabrication under intense heat, such as casting metal or firing porcelain, that would not be po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridge (dentistry)
A bridge is a fixed dental restoration (a fixed dental prosthesis) used to replace one or more missing teeth by joining an artificial tooth definitively to adjacent teeth or dental implants. Definitions Fixed bridge: A dental prosthesis that is definitively attached to natural teeth and replaces missing teeth. Abutment: The tooth that supports and retains a dental prosthesis. Pontic: The artificial tooth that replaces a missing natural tooth. Retainer: The component attached to the abutment for retention of the prosthesis. Retainers can be major or minor. Unit: Pontics and abutment teeth are referred to as units. The total number of units in a bridge is equal to the number of pontics plus the number of abutment teeth. Saddle: The area on the alveolar ridge which is edentulous where at least one missing tooth is to be reinstated. Connector: Joins the pontic to the retainer or two retainers together. Connectors may be fixed or movable. Span: The length of the alveolar ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthodontic
Orthodontics is a dentistry specialty that addresses the diagnosis, prevention, management, and correction of mal-positioned teeth and jaws, and misaligned bite patterns. It may also address the modification of facial growth, known as dentofacial orthopedics. Abnormal alignment of the teeth and jaws is very common. Nearly 50% of the developed world's population, according to the American Association of Orthodontics, has malocclusions severe enough to benefit from orthodontic treatment: although this figure decreases to less than 10% according to the same AAO statement when referring to medically necessary orthodontics. However, conclusive scientific evidence for the health benefits of orthodontic treatment is lacking, although patients with completed orthodontic treatment have reported a higher quality of life than that of untreated patients undergoing orthodontic treatment. Treatment may require several months to a few years, and entails using dental braces and other appliances t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, Zinc metabolism, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. ZnO is a wide-band gap semiconductor of the II-VI semiconductor, II-VI semiconductor group. The native doping (semiconductor), doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility, wide band gap, and strong room-temperature luminescence. Those properties make ZnO valuable for a variety of emerging applications: transparent electrodes in liquid crystal displays, energy-saving o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dental Restoration
Dental restoration, dental fillings, or simply fillings are treatments used to restore the function, integrity, and morphology of missing tooth structure resulting from caries or external trauma as well as to the replacement of such structure supported by dental implants. They are of two broad types—''direct'' and ''indirect''—and are further classified by location and size. A root canal filling, for example, is a restorative technique used to fill the space where the dental pulp normally resides. Tooth preparation Restoring a tooth to good form and function requires two steps: # preparing the tooth for placement of restorative material or materials, and # placement of these materials. The process of preparation usually involves cutting the tooth with a rotary dental handpiece and dental burrs, a dental laser, or though air abrasion to make space for the planned restorative materials and to remove any dental decay or portions of the tooth that are structurally unsound. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other " phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |