|
PIN1
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 is an enzyme that in humans is encoded by the ''PIN1'' gene. Pin 1, or peptidyl-prolyl cis/trans isomerase (PPIase), isomerizes only phospho-Serine/Threonine-Proline motifs. The enzyme binds to a subset of proteins and thus plays a role as a post phosphorylation control in regulating protein function. Studies have shown that the deregulation of Pin1 may play a pivotal role in various diseases. Notably, the up-regulation of Pin1 is implicated in certain cancers, and the down-regulation of Pin1 is implicated in Alzheimer's disease. Inhibitors of Pin1 may have therapeutic implications for cancer and immune disorders. Discovery The gene encoding Pin1 was identified in 1996 as a result of a genetic/biochemical screen for proteins involved in mitotic regulation. It was found to be essential for cell division in some organisms. By 1999, however, it was apparent that Pin1 knockout mice had a surprisingly mild phenotype, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PIN Proteins
PIN proteins are integral membrane proteins in plants that transport the anionic form of the hormone auxin across membranes. The discovery of the initial member of the PIN gene family, PIN1, occurred through the identification of the pin-formed1 (pin1) mutation in ''Arabidopsis thaliana''. This mutation led to a stem that lacked almost all organs, including leaves and flowers. Most of the PIN proteins (e.g. PIN1/2/3/4/7 in the model plant ''Arabidopsis thaliana'') localize at the plasma membrane (PM) where they serve as secondary active transporters involved in the efflux of auxin. The PM-localized PIN proteins show asymmetrical localizations on the membrane and are, therefore, responsible for polar auxin transport. Some other members of the PIN family (e.g. PIN5 and 8 in ''Arabidopsis'') localize mostly at the ER-membrane or have a dual PM and ER localization (e.g. PIN6 in ''Arabidopsis''). These PIN proteins regulate the partitioning of auxin within the cell. The PM-localize ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WW Domain
The WW domain (also known as the rsp5-domain or WWP repeating structural motif, motif) is a modular protein domain that mediates specific interactions with protein ligands. This domain is found in a number of unrelated signaling and structural proteins and may be repeated up to four times in some proteins. Apart from binding preferentially to proteins that are proline-rich, with particular proline-motifs, [AP]-P-P-[AP]-Y, some WW domains bind to phosphoserine- and phosphothreonine-containing motifs. Structure and ligands The WW domain is one of the smallest protein modules, composed of only 40 amino acids, which mediates specific protein-protein interactions with short proline-rich or proline-containing motifs. Named after the presence of two conserved tryptophans (W), which are spaced 20-22 amino acids apart within the sequence, the WW domain folds into a meandering triple-stranded beta sheet. The identification of the WW domain was facilitated by the analysis of two splice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols: : This equation can be written in several ways that are nearly equivalent that describe the behaviors of various protonated states of ATP, ADP, and the phosphorylated product. As is clear from the equation, a phosphate group per se is not transferred, but a phosphoryl group (PO3-). Phosphoryl is an electrophile. This process and its inverse, dephosphorylation, are common in biology. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. During respiration Phosphorylation is essential to the processes of both anaerobic and aerobic respiration, which involve the production of adenosine triphosphate (ATP), the "high-energy" exc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cdc25
Cdc25 is a dual-specificity phosphatase first isolated from the yeast ''Schizosaccharomyces pombe'' as a cell cycle defective mutant. As with other cell cycle proteins or genes such as Cdc2 and Cdc4, the "cdc" in its name refers to "cell division cycle". Dual-specificity phosphatases are considered a sub-class of protein tyrosine phosphatases. By removing inhibitory phosphate residues from target cyclin-dependent kinases (Cdks), Cdc25 proteins control entry into and progression through various phases of the cell cycle, including mitosis and S ("Synthesis") phase. Function in activating Cdk1 Cdc25 activates cyclin dependent kinases by removing phosphate from residues in the Cdk active site. In turn, the phosphorylation by M-Cdk (a complex of Cdk1 and cyclin B) activates Cdc25. Together with Wee1, M-Cdk activation is switch-like. The switch-like behavior forces entry into mitosis to be quick and irreversible. Cdk activity can be reactivated after dephosphorylation by Cdc25. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dephosphorylation
In biochemistry, dephosphorylation is the removal of a phosphate () group from an organic compound by hydrolysis. It is a reversible post-translational modification. Dephosphorylation and its counterpart, phosphorylation, activate and deactivate enzymes by detaching or attaching phosphoric esters and anhydrides. A notable occurrence of dephosphorylation is the conversion of Adenosine triphosphate, ATP to Adenosine diphosphate, ADP and inorganic phosphate. Dephosphorylation employs a type of hydrolytic enzyme, or hydrolase, which cleaves ester bonds. The prominent hydrolase subclass used in dephosphorylation is phosphatase, which removes phosphate groups by hydrolysing phosphoric acid monoesters into a phosphate ion and a molecule with a free hydroxyl (–OH) group. The reversible phosphorylation-dephosphorylation reaction occurs in every physiological process, making proper function of protein phosphatases necessary for organism viability. Because protein dephosphorylation is a k ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Signalling
In biology, cell signaling (cell signalling in British English) is the process by which a cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all cellular life in both prokaryotes and eukaryotes. Typically, the signaling process involves three components: the signal, the receptor, and the effector. In biology, signals are mostly chemical in nature, but can also be physical cues such as pressure, voltage, temperature, or light. Chemical signals are molecules with the ability to bind and activate a specific receptor. These molecules, also referred to as ligands, are chemically diverse, including ions (e.g. Na+, K+, Ca2+, etc.), lipids (e.g. steroid, prostaglandin), peptides (e.g. insulin, ACTH), carbohydrates, glycosylated proteins (proteoglycans), nucleic acids, etc. Peptide and lipid ligands are particularly important, as most hormones belong to these classes of chemicals. Peptides are usually polar, hydrophilic molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GSK3
Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen synthase (GS), GSK-3 has since been identified as a protein kinase for over 100 different proteins in a variety of different pathways. In mammals, including humans, GSK-3 exists in two isozymes encoded by two homologous genes GSK-3α (GSK3A) and GSK-3β (GSK3B). GSK-3 has been the subject of much research since it has been implicated in a number of diseases, including type 2 diabetes, Alzheimer's disease, inflammation, cancer, addiction and bipolar disorder. GSK-3 is a serine/threonine protein kinase that phosphorylate either threonine or serine, and this phosphorylation controls a variety of biological activities, such as glycogen metabolism, cell signaling, cellular transport, and others. GS inhibition by GSK-3β leads to a decrease in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclin-dependent Kinase
Cyclin-dependent kinases (CDKs) are a predominant group of serine/threonine protein kinases involved in the regulation of the cell cycle and its progression, ensuring the integrity and functionality of cellular machinery. These regulatory enzymes play a crucial role in the regulation of eukaryotic cell cycle and Eukaryotic transcription, transcription, as well as DNA repair, metabolism, and Epigenetics, epigenetic regulation, in response to several extracellular and intracellular signals. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. The catalytic activities of CDKs are regulated by interactions with CDK inhibitors (CKIs) and regulatory subunits known as cyclins. Cyclins have no enzymatic activity themselves, but they become active once they bind to CDKs. Without cyclin, CDK is less active than in the cyclin-CDK heterodimer complex. CDKs phosphorylate proteins on serine (S) or threonine (T) residues. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAPK
A mitogen-activated protein kinase (MAPK or MAP kinase) is a type of serine/threonine-specific protein kinases involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines. They regulate cell functions including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis. MAP kinases are found in eukaryotes only, but they are fairly diverse and encountered in all animals, fungi and plants, and even in an array of unicellular eukaryotes. MAPKs belong to the CMGC (CDK/MAPK/GSK3/CLK) kinase group. The closest relatives of MAPKs are the cyclin-dependent kinases (CDKs). Discovery The first mitogen-activated protein kinase to be discovered was ERK1 ( MAPK3) in mammals. Since ERK1 and its close relative ERK2 ( MAPK1) are both involved in growth factor signaling, the family was termed "mitogen-activated". With the discovery of other members, even from distant organis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
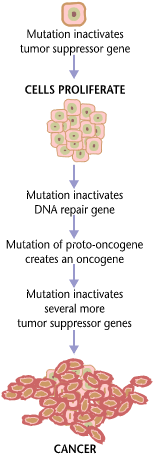
Oncogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Proliferation
Cell proliferation is the process by which ''a cell grows and divides to produce two daughter cells''. Cell proliferation leads to an exponential increase in cell number and is therefore a rapid mechanism of tissue growth. Cell proliferation requires both cell growth and cell division to occur at the same time, such that the average size of cells remains constant in the population. Cell division can occur without cell growth, producing many progressively smaller cells (as in cleavage of the zygote), while cell growth can occur without cell division to produce a single larger cell (as in growth of neurons). Thus, cell proliferation is not synonymous with either cell growth or cell division, despite these terms sometimes being used interchangeably. Stem cells undergo cell proliferation to produce proliferating "transit amplifying" daughter cells that later differentiate to construct tissues during normal development and tissue growth, during tissue regeneration after da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KPNA1
Importin subunit alpha-5 is a protein that in humans is encoded by the ''KPNA1'' gene. Interactions Importin subunit alpha-5 has been shown to interact with KPNB1 Importin subunit beta-1 is a protein that in humans is encoded by the ''KPNB1'' gene. Function Nucleocytoplasmic transport, a signal- and energy-dependent process, takes place through nuclear pore complexes embedded in the nuclear envelope. T ... and UBR5. References Further reading * * * * * * * * * * * * * * * * * * * Armadillo-repeat-containing proteins {{gene-3-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |