|
Oxidase
In biochemistry, an oxidase is an oxidoreductase (any enzyme that catalyzes a redox reaction) that uses dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydrogen peroxide (H2O2). Some oxidation reactions, such as those involving monoamine oxidase or xanthine oxidase, typically do not involve free molecular oxygen. The oxidases are a subclass of the oxidoreductases. The use of dioxygen is the only unifying feature; in the EC classification, these enzymes are scattered in many categories. Examples An important example is EC 7.1.1.9 cytochrome c oxidase, the key enzyme that allows the body to employ oxygen in the generation of energy and the final component of the electron transfer chain. Other examples are: * EC 1.1.3.4 Glucose oxidase * EC 1.4.3.4 Monoamine oxidase * EC 1.14.-.- Cytochrome P450 oxidase * EC 1.6.3.1 NADPH oxidase * EC 1.17.3.2 Xanthine oxidase * EC 1.1.3.8 L-gulonolactone oxidase * EC 1. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C Oxidase
The enzyme cytochrome c oxidase or Complex IV (was , now reclassified as a translocasEC 7.1.1.9 is a large transmembrane protein complex found in bacteria, archaea, and the mitochondria of eukaryotes. It is the last enzyme in the Cellular respiration, respiratory electron transport chain of cell (biology), cells located in the membrane. It receives an electron from each of four cytochrome c molecules and transfers them to one oxygen molecule and four protons, producing two molecules of water. In addition to binding the four protons from the inner aqueous phase, it transports another four protons across the membrane, increasing the transmembrane difference of proton electrochemical potential, which the ATP synthase then uses to synthesize Adenosine triphosphate, ATP. Structure The complex The complex is a large integral membrane protein composed of several Cofactor (biochemistry)#Metal ions, metal prosthetic sites and 13 protein subunits in mammals. In mammals, ten subunits a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome Oxidase
The enzyme cytochrome c oxidase or Complex IV (was , now reclassified as a translocasEC 7.1.1.9 is a large transmembrane protein complex found in bacteria, archaea, and the mitochondria of eukaryotes. It is the last enzyme in the respiratory electron transport chain of cells located in the membrane. It receives an electron from each of four cytochrome c molecules and transfers them to one oxygen molecule and four protons, producing two molecules of water. In addition to binding the four protons from the inner aqueous phase, it transports another four protons across the membrane, increasing the transmembrane difference of proton electrochemical potential, which the ATP synthase then uses to synthesize ATP. Structure The complex The complex is a large integral membrane protein composed of several metal prosthetic sites and 13 protein subunits in mammals. In mammals, ten subunits are nuclear in origin, and three are synthesized in the mitochondria. The complex contain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyphenol Oxidase
Polyphenol oxidase (PPO; also polyphenol oxidase i, chloroplastic), an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule. PPO may accept monophenols and/or ''o''-diphenols as substrates. The enzyme works by catalyzing the ''o''- hydroxylation of monophenol molecules in which the benzene ring contains a single hydroxyl substituent to ''o''-diphenols (phenol molecules containing two hydroxyl substituents at the 1, 2 positions, with no carbon between). It can also further catalyse the oxidation of ''o''-diphenols to produce ''o''-quinones. PPO catalyses the rapid polymerization of ''o''-quinones to produce black, brown or red pigments (polyphenols) that cause fruit browning. The amino acid tyrosine contains a single phenolic ring that may be oxidised by the action of PPOs to form ''o''-quinone. Hence, PPOs may also be referred to as tyrosinases. Common foods producing the enzyme include mushrooms ('' Agaricus bisporus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysyl Oxidase
Lysyl oxidase (LOX), also known as protein-lysine 6-oxidase, is an enzyme that, in humans, is encoded by the ''LOX'' gene. It catalyzes the conversion of lysine residues into its aldehyde derivative allysine. Allysine form cross-links in extracellular matrix proteins. Inhibition of lysyl oxidase can cause osteolathyrism, but, at the same time, its upregulation by tumor cells may promote metastasis of the existing tumor, causing it to become malignant and cancerous. Structure In the yeast species ''Pichia pastoris'', lysyl oxidase constitutes a homodimeric structure. Each monomer consists of an active site that includes a Cu(II) atom, coordinated by three histidine residues, as well as 2,4,5-trihydroxyphenylalanine quinone (TPQ), a crucial cofactor. In humans, the LOX gene is located on chromosome 5 q23.3-31.2. The DNA sequence encodes a polypeptide of 417 amino acids, the first 21 residues of which constitute a signal peptide, with a weight of approximately 32 kDa. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase
Monoamine oxidases (MAO) () are a family of enzymes that catalyze the oxidation of monoamines, employing oxygen to clip off their amine group. They are found bound to the outer membrane of mitochondria in most cell types of the body. The first such enzyme was discovered in 1928 by Mary Bernheim in the liver and was named tyramine oxidase. The MAOs belong to the protein family of flavin-containing amine oxidoreductases. MAOs are important in the breakdown of monoamines ingested in food, and also serve to inactivate monoamine neurotransmitters. Because of the latter, they are involved in a number of psychiatric and neurological diseases, some of which can be treated with monoamine oxidase inhibitors (MAOIs) which block the action of MAOs. Subtypes and tissue distribution In humans there are two types of MAO: MAO-A and MAO-B. * Both are found in neurons and astroglia. * Outside the central nervous system: ** MAO-A is also found in the liver, pulmonary vascular end ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
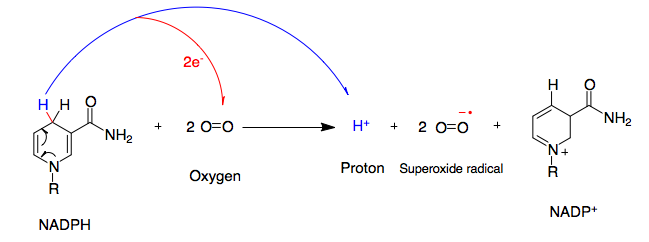
NADPH Oxidase
NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) is a membrane-bound enzyme complex that faces the extracellular space. It can be found in the plasma membrane as well as in the membranes of phagosomes used by neutrophil white blood cells to engulf microorganisms. Human Protein isoform, isoforms of the catalytic component of the complex include NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1, and DUOX2. Reaction NADPH oxidase catalyzes the production of a superoxide free radical by transferring one electron to oxygen from NADPH. : Types In mammals, NADPH oxidase is found in two types: one in white blood cells (neutrophilic) and the other in Blood vessel, vascular cells, differing in biochemical structure and functions. Neutrophilic NADPH oxidase produces superoxide almost instantaneously, whereas the vascular enzyme produces superoxide in minutes to hours. Moreover, in white blood cells, superoxide has been found to transfer electrons across the membrane to extracellula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
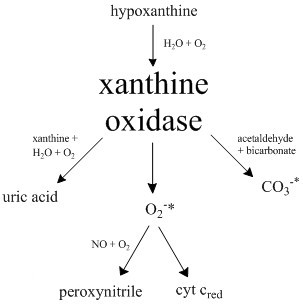
Xanthine Oxidase
Xanthine oxidase (XO or XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. "XDH xanthine dehydrogenase" Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 xanthine + H2O2 * xanthine + H2O + O2 uric acid + H2O2 * Xanthine oxidase ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-gulonolactone Oxidase
L-Gulonolactone oxidase ( ECbr>1.1.3.8 is an enzyme that produces vitamin C. It is expressed in most mammals, but is non-functional in Haplorrhini (a suborder of primates, including humans), in some bats, and in guinea pigs. It catalyzes the reaction of L-gulono-1,4-lactone with oxygen to form L-xylo-hex-3-gulonolactone (2-keto-gulono-γ-lactone) and hydrogen peroxide. It uses FAD as a cofactor. The L-xylo-hex-3-gulonolactone then converts to ascorbic acid spontaneously, without enzymatic action. The structure of L-gulonolactone oxidase in rats helps identify characteristics of this enzyme. Gulonolactone oxidase deficiency The non-functional gulonolactone oxidase pseudogene (''GULOP'') was mapped to human chromosome 8p21, which corresponds to an evolutionarily conserved segment on either porcine chromosome 4 (SSC4) or 14 (SSC14). GULO produces the precursor to ascorbic acid, which spontaneously converts to the vitamin itself. The loss of activity of the gene encoding L-gul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose Oxidase
The glucose oxidase enzyme (GOx or GOD) also known as notatin (EC number 1.1.3.4) is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present. Glucose oxidase is widely used for the determination of free glucose in body fluids (medical testing), in vegetal raw material, and in the food industry. It also has many applications in biotechnologies, typically enzyme assays for biochemistry including biosensors in nanotechnologies. It was first isolated by Detlev Müller in 1928 from ''Aspergillus niger''. Function Several species of fungi and insects synthesize glucose oxidase, which produces hydrogen peroxide, which kills bacteria. Notatin, extracted from antibacterial cultures of '' Penicillium notatum'', was originally named Penicillin A, but was renamed to avoid confusion with penicillin. Not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. Many of the enzymes in the electron transport chain are embedded within the membrane. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redox reactions are driven by the difference in the Gibbs free energy of reactants and products. The free energy released when a higher ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |