|
Osmotic
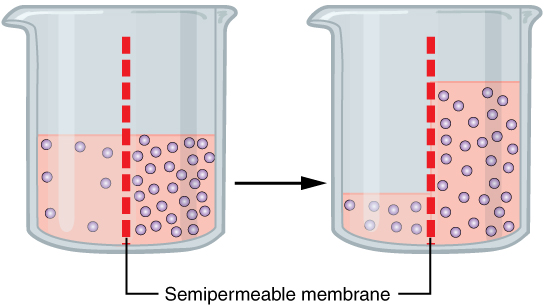
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane (permeable to the solvent, but not the solute) separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to prevent net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process in biological systems, as biological membranes are semipermeable. In general, thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Potential
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure and matrix effects such as capillary action (which is caused by surface tension). The concept of water potential has proved useful in understanding and computing water movement within plants, animals, and soil. Water potential is typically expressed in potential energy per unit volume and very often is represented by the Greek letter ψ. Water potential integrates a variety of different potential drivers of water movement, which may operate in the same or different directions. Within complex biological systems, many potential factors may be operating simultaneously. For example, the addition of solutes lowers the potential (negative vector), while an increase in pressure increases the potential (positive vector). If the flow is not restricted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a Solution (chemistry), solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in its pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it was not separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until ''osmotic equilibrium'' is attained. Theory and measurement Jacobus Henricus van 't Hoff, Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colligative Properties
In chemistry, colligative properties are those properties of solutions that depend on the ratio of the number of solute particles to the number of solvent particles in a solution, and not on the nature of the chemical species present. The number ratio can be related to the various units for concentration of a solution such as molarity, molality, normality (chemistry), etc. The assumption that solution properties are independent of nature of solute particles is exact only for ideal solutions, which are solutions that exhibit thermodynamic properties analogous to those of an ideal gas, and is approximate for dilute real solutions. In other words, colligative properties are a set of solution properties that can be reasonably approximated by the assumption that the solution is ideal. Only properties which result from the dissolution of a nonvolatile solute in a volatile liquid solvent are considered.KL Kapoor ''Applications of Thermodynamics'' Volume 3 They are essentially s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Turgor Pressure
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall. It is also called ''hydrostatic pressure'', and is defined as the pressure in a fluid measured at a certain point within itself when at equilibrium. Generally, turgor pressure is caused by the osmotic flow of water and occurs in plants, fungi, and bacteria. The phenomenon is also observed in protists that have cell walls. This system is not seen in animal cells, as the absence of a cell wall would cause the cell to lyse when under too much pressure. The pressure exerted by the osmotic flow of water is called turgidity. It is caused by the osmotic flow of water through a selectively permeable membrane. Movement of water through a semipermeable membrane from a volume with a low solute concentration to one with a higher solute concentration is called osmotic flow. In plants, this entails the water moving from the low concentration solute outside the cell into the cell's vacuole. Mec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semipermeable Membrane
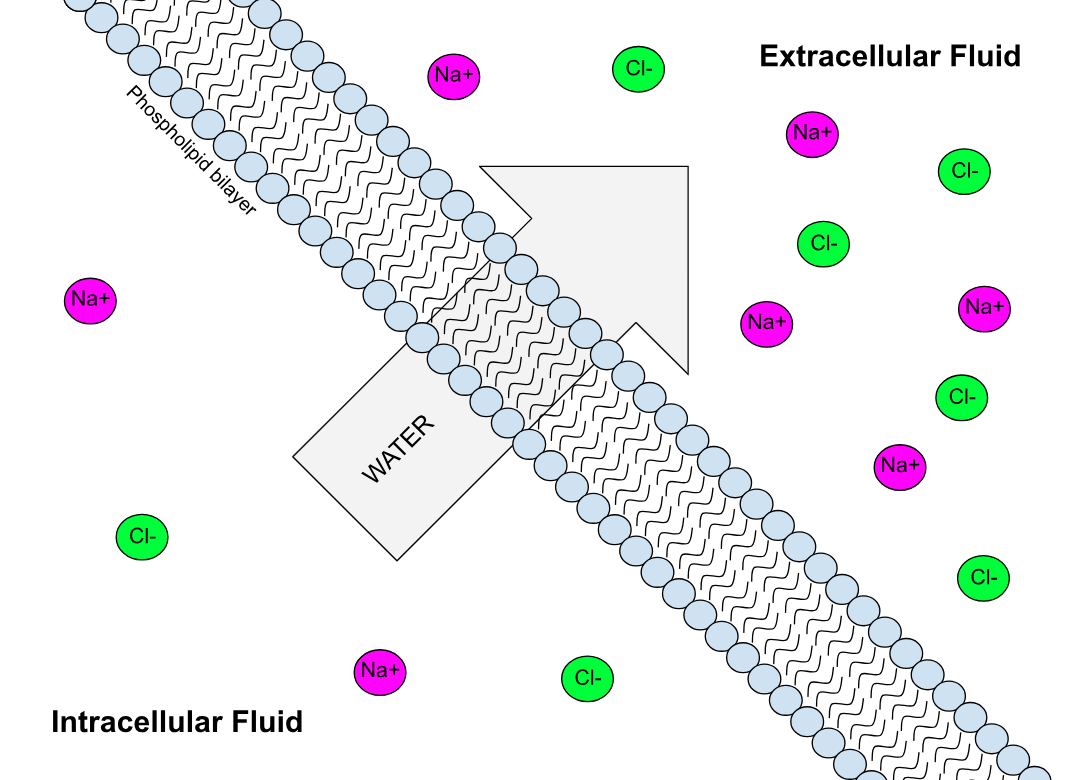
Semipermeable membrane is a type of synthetic or biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes Phospholipid bilayer A phospholipid bilayer is an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selectively Permeable Membrane
Semipermeable membrane is a type of Chemical synthesis, synthetic or Biological membrane, biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg. Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane. Biological membranes Phospholipid bilayer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moritz Traube
Moritz Traube (12 February 1826 – 28 June 1894) was a German chemist and universal private scholar. Traube worked on chemical, biochemical, medical, physiological, pathophysiological problems. He was engaged in hygienics, physical chemistry and basic chemical research. Although he was never a staff member of a university and earned his living as a wine merchant, he was able to refute theories of his leading contemporaries, including Justus von Liebig, Louis Pasteur, Felix Hoppe-Seyler and Julius Sachs, and to develop significant theories of his own with solid experimental foundations. The chemistry of oxygen and its significance to the organism were the central objects of his research and provided the common thread uniting almost all of his scientific activity. Moritz Traube was a younger brother of the famous Berlin physician Ludwig Traube (physician), the co-founder of the German experimental pathology. A son, Wilhelm Traube, evolved a process of purine synthesis. Hermann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of all life, forms of life. Every cell consists of cytoplasm enclosed within a Cell membrane, membrane; many cells contain organelles, each with a specific function. The term comes from the Latin word meaning 'small room'. Most cells are only visible under a light microscope, microscope. Cells Abiogenesis, emerged on Earth about 4 billion years ago. All cells are capable of Self-replication, replication, protein synthesis, and cell motility, motility. Cells are broadly categorized into two types: eukaryotic cells, which possess a Cell nucleus, nucleus, and prokaryotic, prokaryotic cells, which lack a nucleus but have a nucleoid region. Prokaryotes are single-celled organisms such as bacteria, whereas eukaryotes can be either single-celled, such as amoebae, or multicellular organism, multicellular, such as some algae, plants, animals, and fungi. Eukaryotic cells contain organelles including Mitochondrion, mitochondria, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
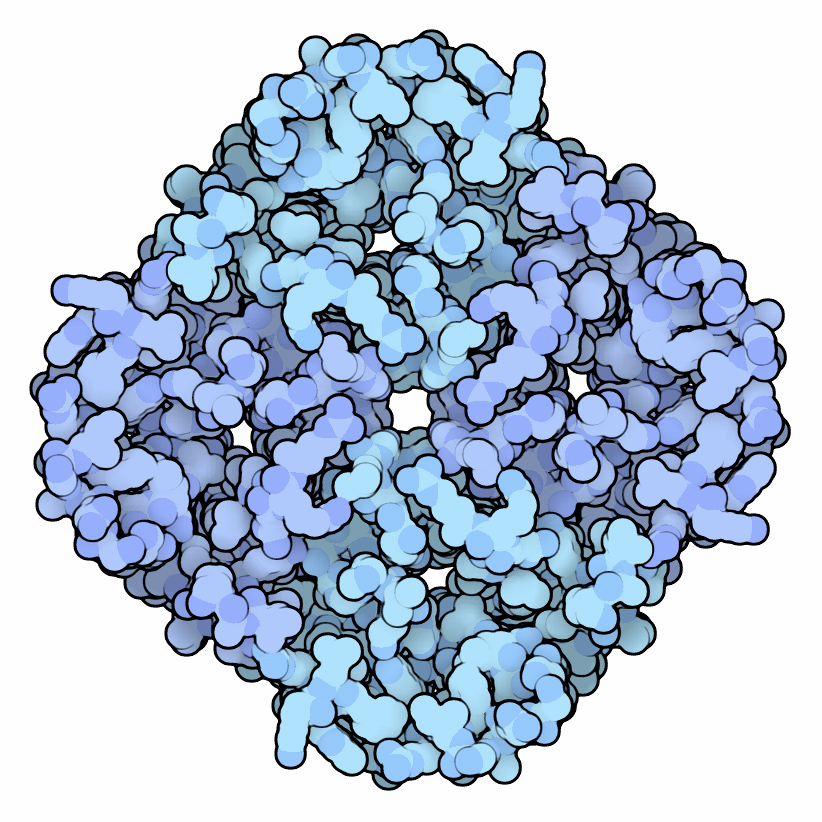
Aquaporin
Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a variety of different bacteria, fungi, animal and plant cells contain aquaporins through which water can flow more rapidly into and out of the cell than by diffusing through the phospholipid bilayer. Aquaporins have six membrane-spanning alpha helical domains with both carboxylic and amino terminals on the cytoplasmic side. Two hydrophobic loops contain conserved asparagine– proline– alanine ("NPA motif") which form a barrel surrounding a central pore-like region that contains additional protein density. Because aquaporins are usually always open and are prevalent in just about every cell type, this leads to a misconception that water readily passes through the cell membrane down its concentration gradient. Water can pass through th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
0307 Osmosis
3 (three) is a number, numeral and digit. It is the natural number following 2 and preceding 4, and is the smallest odd prime number and the only prime preceding a square number. It has religious and cultural significance in many societies. Evolution of the Arabic digit The use of three lines to denote the number 3 occurred in many writing systems, including some (like Roman and Chinese numerals) that are still in use. That was also the original representation of 3 in the Brahmic (Indian) numerical notation, its earliest forms aligned vertically. However, during the Gupta Empire the sign was modified by the addition of a curve on each line. The Nāgarī script rotated the lines clockwise, so they appeared horizontally, and ended each line with a short downward stroke on the right. In cursive script, the three strokes were eventually connected to form a glyph resembling a with an additional stroke at the bottom: ३. The Indian digits spread to the Caliphate in the 9th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nouvelles Recherches Sur L Endosmose Et 125
Nouvelles (; ) is a sub-municipality of the city of Mons located in the province of Hainaut, Wallonia, Belgium. It was a separate municipality until 1977. On 1 January 1977, it was merged Mergers and acquisitions (M&A) are business transactions in which the ownership of a company, business organization, or one of their operating units is transferred to or consolidated with another entity. They may happen through direct absorpt ... into Mons. References Sub-municipalities of Mons, Belgium Former municipalities of Hainaut (province) {{Hainaut-geo-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry Watts (chemist)
Henry Watts (1815–1884) was an English chemist. Life He was born in London on 20 January 1815. He went to a private school, and was articled at the age of fifteen as an architect and surveyor; but went on to support himself by teaching, chiefly mathematical, privately and at a school. He then attended University College, London. In 1841 he graduated B.A. in the University of London. In 1846 he became assistant to George Fownes, professor of practical chemistry at University College, and occupied this post, after Fownes's death in 1849, until 1857, under Professor Alexander William Williamson. Having an impediment in speech he found himself unable to obtain a professorship, and worked on the literature of chemistry. In 1847 he was elected fellow of the Chemical Society. On 17 December 1849 he was elected editor of the Chemical Society's ''Journal'', and about the beginning of 1860 he also became librarian to the society. Early in 1871 it was decided to print in the society's j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |