|
Omecamtiv Mecarbil
Omecamtiv mecarbil ( INN), previously referred to as CK-1827452, is a cardiac-specific myosin activator. It is being studied for a potential role in the treatment of left ventricular systolic heart failure. Systolic heart failure involves a loss of effective actin-myosin cross bridges in the myocytes (heart muscle cells) of the left ventricle, which leads to a decreased ability of the heart to move blood through the body. This causes peripheral edema (blood pooling), which the sympathetic nervous system tries to correct by overstimulating the cardiac myocytes, leading to left ventricular hypertrophy, another characteristic of chronic heart failure. Current inotropic therapies work by increasing the force of cardiac contraction, such as through calcium conduction or modulating adrenoreceptors. But these are limited by adverse events, including arrhythmias related to increased myocardial oxygen consumption, desensitization of adrenergic receptors, and altering intracellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
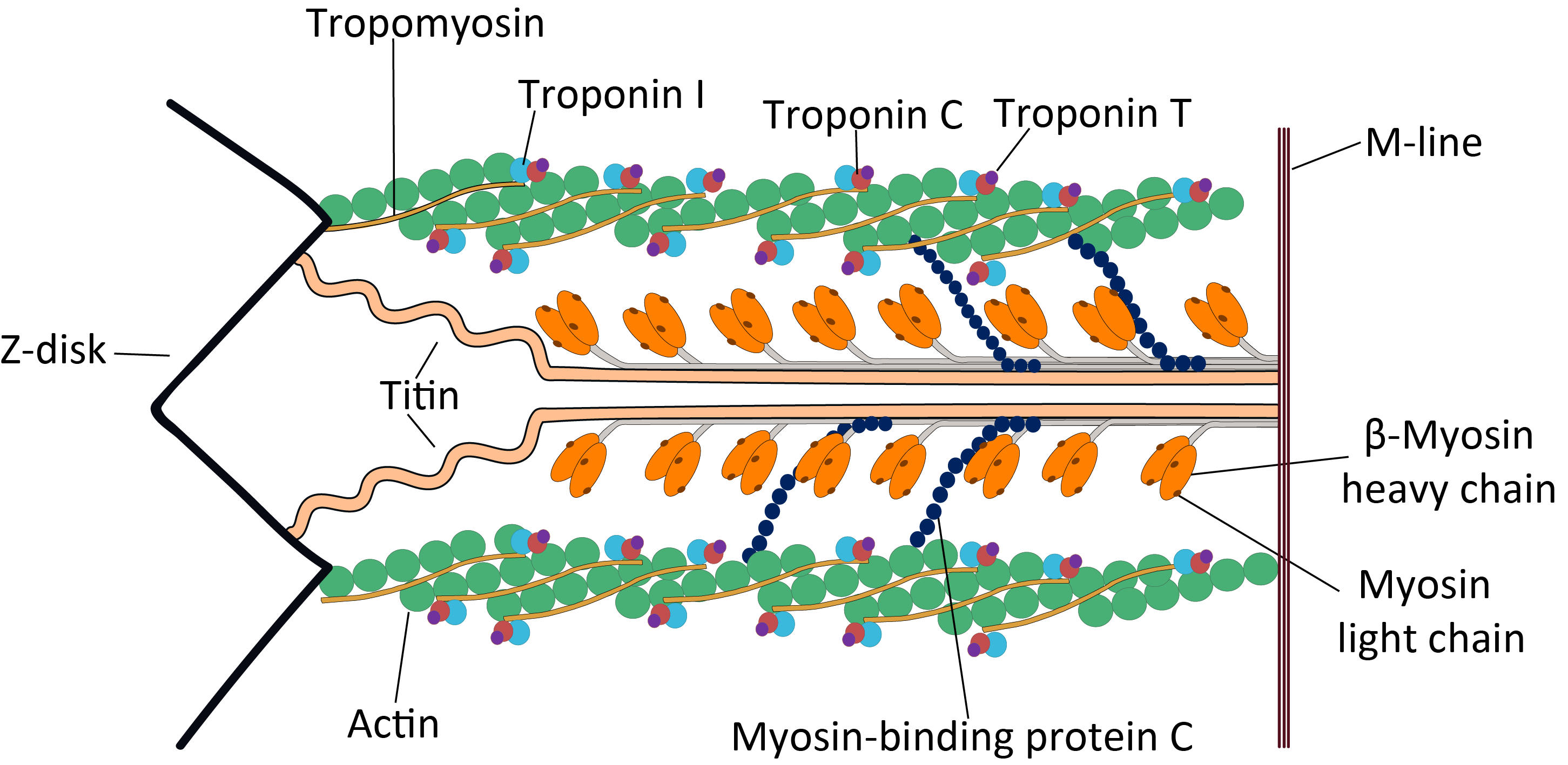
Sarcomere
A sarcomere (Greek σάρξ ''sarx'' "flesh", μέρος ''meros'' "part") is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells (called muscle fibers or myofibers) which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma. Two of the important proteins are myosin, which forms the thick filament, and actin, which forms the thin filament. Myosin has a long, fibrous tail and a globular head, which binds to actin. The myosin head also binds to ATP, which is the source of energy for muscle movement. Myo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AMGEN
Amgen Inc. (formerly Applied Molecular Genetics Inc.) is an American multinational biopharmaceutical company headquartered in Thousand Oaks, California. One of the world's largest independent biotechnology companies, Amgen was established in Thousand Oaks, California, in 1980.Baker, Pam (2002). ''Thousand Oaks Westlake Village: A Contemporary Portrait''. Community Communications, Inc. Page 37. . Amgen's Thousand Oaks staff in 2017 numbered 5,125 (7.5% of total city employment) and included hundreds of scientists, making Amgen the largest employer in Ventura County. Focused on molecular biology and biochemistry, its goal is to provide a healthcare business based on recombinant DNA technology. In 2018, the company's largest selling product lines were Neulasta, an immunostimulator used to prevent infections in patients undergoing cancer chemotherapy and Enbrel, a tumor necrosis factor blocker used in the treatment of rheumatoid arthritis and other autoimmune diseases. Othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track Designation
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast Track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast Track is one of five Food and Drug Administration (FDA) approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and Regenerative Medicine Advanced Therapy. Fast Track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs, but involves such things as regulating lasers, cellular phones, and condoms, as well as control of disease in contexts v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomized Clinical Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing standard of care; these are then termed the 'experimenta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exercise Intolerance
Exercise intolerance is a condition of inability or decreased ability to perform physical exercise at the normally expected level or duration for people of that age, size, sex, and muscle mass. It also includes experiences of unusually severe post-exercise pain, fatigue, nausea, vomiting or other negative effects. Exercise intolerance is not a disease or syndrome in and of itself, but can result from various disorders. In most cases, the specific reason that exercise is not tolerated is of considerable significance when trying to isolate the cause down to a specific disease. Dysfunctions involving the pulmonary, cardiovascular or neuromuscular systems have been frequently found to be associated with exercise intolerance, with behavioural causes also playing a part. Signs and symptoms Exercise in this context means physical activity, not specifically exercise in a fitness program. For example, a person with exercise intolerance after a heart attack may not be able to sustain the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase III Clinical Trial
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over seve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desensitization (medicine)
In medicine, desensitization is a method to reduce or eliminate an organism's negative reaction to a substance or stimulus. In pharmacology, ''drug desensitization'' refers to two related concepts. First, desensitization may be equivalent to drug tolerance and refers to subjects' reactions (positive or negative) to a drug reducing following its repeated use. This is a macroscopic, organism-level effect and differs from the second meaning of desensitization, which refers to a biochemical effect where individual receptors become less responsive after repeated application of an agonist. This may be mediated by phosphorylation, for instance by beta adrenoceptor kinase at the beta adrenoceptor. Application to allergies For example, if a person with diabetes mellitus has a bad allergic reaction to taking a full dose of beef insulin, the person is given a very small amount of the insulin at first, so small that the person has no adverse reaction or very limited symptoms as a result. Ove ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heart Rate
Heart rate (or pulse rate) is the frequency of the heartbeat measured by the number of contractions (beats) of the heart per minute (bpm). The heart rate can vary according to the body's physical needs, including the need to absorb oxygen and excrete carbon dioxide, but is also modulated by numerous factors, including, but not limited to, genetics, physical fitness, stress or psychological status, diet, drugs, hormonal status, environment, and disease/illness as well as the interaction between and among these factors. It is usually equal or close to the pulse measured at any peripheral point. The American Heart Association states the normal resting adult human heart rate is 60–100 bpm. Tachycardia is a high heart rate, defined as above 100 bpm at rest. Bradycardia is a low heart rate, defined as below 60 bpm at rest. When a human sleeps, a heartbeat with rates around 40–50 bpm is common and is considered normal. When the heart is not beating in a regular pattern, this i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)


