|
Oligosaccharyltransferase
Oligosaccharyltransferase or OST () is a membrane protein complex that transfers a 14-sugar oligosaccharide from dolichol to nascent protein. It is a type of glycosyltransferase. The sugar Glc3Man9GlcNAc2 (where Glc=Glucose, Man= Mannose, and GlcNAc= ''N''-acetylglucosamine) is attached to an asparagine (Asn) residue in the sequence Asn-X- Ser or Asn-X- Thr where X is any amino acid except proline. This sequence is called a glycosylation '' sequon.'' The reaction catalyzed by OST is the central step in the ''N''-linked glycosylation pathway. Location OST is a component of the translocon in the endoplasmic reticulum (ER) membrane. A lipid-linked core-oligosaccharide is assembled at the membrane of the endoplasmic reticulum and transferred to selected asparagine residues of nascent polypeptide chains by the oligosaccharyl transferase complex. The active site of OST is located about 4 nm from the lumenal face of the ER membrane. It usually acts during translation a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OST4
In molecular biology, OST4 (Dolichyl-diphosphooligosaccharide—protein glycosyltransferase subunit 4) is a subunit of the oligosaccharyltransferase complex. OST4 is a very short, approximately 30 amino acids, protein found from fungi to vertebrates. It appears to be an integral membrane protein that mediates the en bloc transfer of a pre-assembled high-mannose oligosaccharide onto asparagine residues of nascent polypeptides as they enter the lumen of the rough endoplasmic reticulum The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little n .... References {{InterPro content, IPR018943 Protein families Chi, J.H., Roos, J. and N. Dean. (1996) The OST4 gene of Saccharomyces cerevisiae encodes an unusually small protein required for normal levels of oligosaccharyltransferase activity J. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asparagine
Asparagine (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC. The one-letter symbol N for asparagine was assigned arbitrarily, with the proposed mnemonic asparagi''N''e; History Asparagine was first isolated in 1806 in a crystalline form by French chemists Louis Nicolas Vauquelin and Pierre Jean Robiquet (then a young assistant). It was isolated from asparagus juice, in which it is abundant, hence the chosen name. It was the first amino acid to be isolated. Three years later, in 1809, Pierre Jean Robiquet identified a substance from l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
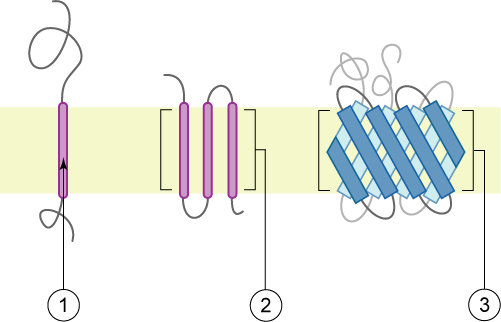
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (Transmembrane protein, transmembrane) or associate with one or the other side of a membrane (Integral monotopic protein, integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct (Native state, native) Protein structure, conformation of the protein in isolation from its native ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OST PM-1
OST may refer to: Music * Original soundtrack, recorded sound accompanying a production such as a film * O.S.T., an alias of electronic musician Chris Douglas * ''O.S.T.'' (album), by the People Under the Stairs * OS/T (album) by S-type Science and technology * Object Storage Target in computing, used by the Lustre file system an others * Oligosaccharyltransferase, an enzyme * Open-space technology, for organising meetings * Open Systems Theory, a form of sociotechnical systems pioneered by Fred Emery * Orbit stabiliser theorem in mathematics * Offline Storage Table, a Microsoft file format * OST Family (organic solute transporter) of genes * Origins Space Telescope, a space telescope mission * Open Source Threat, any software demonstrating a device vulnerability that is open-source Organizations * Office of Science and Technology, a British government agency * Office of Secure Transportation, a US government agency * Order of the Solar Temple, French esoteric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the Polymer backbone, main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a Derivative (chemistry), derivative of a carboxylic acid () with the hydroxyl group () replaced by an amino group (); or, equivalently, an acyl group, acyl (alkanoyl) group () joined to an amino group. Common amides are formamide (), acetamide (), benzamide (), and dimethylformamide (). Some uncommon examples of amides are ''N''-chloroacetamide () and chloroformamide (). Amides are qualified as primary (chemistry), primary, secondary (chemistry), secondary, and tertiary (chemistry), tertiary according to the number of acyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular mass.'' The name is composed of Greek elements '' oligo-'', "a few" and '' -mer'', "parts". An adjective form is ''oligomeric''. The oligomer concept is contrasted to that of a polymer, which is usually understood to have a large number of units, possibly thousands or millions. However, there is no sharp distinction between these two concepts. One proposed criterion is whether the molecule's properties vary significantly with the removal of one or a few of the units. An oligomer with a specific number of units is referred to by the Greek prefix denoting that number, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Folding
Protein folding is the physical process by which a protein, after Protein biosynthesis, synthesis by a ribosome as a linear chain of Amino acid, amino acids, changes from an unstable random coil into a more ordered protein tertiary structure, three-dimensional structure. This structure permits the protein to become biologically functional or active. The folding of many proteins begins even during the translation of the polypeptide chain. The amino acids interact with each other to produce a well-defined three-dimensional structure, known as the protein's native state. This structure is determined by the amino-acid sequence or primary structure. The correct three-dimensional structure is essential to function, although some parts of functional proteins Intrinsically unstructured proteins, may remain unfolded, indicating that protein dynamics are important. Failure to fold into a native structure generally produces inactive proteins, but in some instances, misfolded proteins have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Posttranslational Modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translate mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can expand the chemical set of the 22 amino acids by changing an existing functional group or adding a new one such as phosphate. Phosphorylation is highly effective for controlling the enzyme activity and is the most common change after translation. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (biology)
In biology, translation is the process in living Cell (biology), cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in the addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later protein folding, folds into an Activation energy, active protein and performs its functions in the cell. The polypeptide can also start folding during protein synthesis. The ribosome facilitates decoding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gunnar Von Heijne
Professor Nils Gunnar Hansson von Heijne (born 10 June 1951 in Gothenburg) is a Swedish scientist working on signal peptides, membrane proteins and bioinformatics at the Stockholm Center for Biomembrane Research at Stockholm University. Education Von Heijne graduated 1975 with a Master of Science degree in chemistry and chemical engineering from the Royal Institute of Technology (KTH).Gunnar von Heijne's CV He then became a doctoral student in at KTH, in a research group focussing on and theoreti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |