|
Oligopeptide
An oligopeptide ('' oligo-'', "a few"), is a peptide consisting of two to twenty amino acids, including dipeptides, tripeptides, tetrapeptides, and other polypeptides. Some of the major classes of naturally occurring oligopeptides include aeruginosins, cyanopeptolins, microcystins, microviridins, microginins, anabaenopeptins, and cyclamides. Microcystins are best studied because of their potential toxicity impact in drinking water. A review of some oligopeptides found that the largest class are the cyanopeptolins (40.1%), followed by microcystins (13.4%). Production Oligopeptide classes are produced by nonribosomal peptides synthases (NRPS), except cyclamides and microviridins are synthesized through ribosomic pathways. Examples Examples of oligopeptides include: * Amanitins - Cyclic peptides taken from carpophores of several different mushroom species. They are potent inhibitors of RNA polymerases in most eukaryotic species, the prevent the production of mRNA and prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. Peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides have an N-terminal (amine group) and C-terminal (carboxyl g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antipain
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases. It has been crystallized in complexes with carboxypeptidase, which is obtained from wheat, and ''Leishmania major'' oligopeptidase B. In both cases, the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease. A study was performed for information on the effect of antipain on the quality of post-thawed ram semen. The results from this experiment concluded that antipain aided in the quality of ram semen by maintaining the sperm mobility. Antipain includes the function to inhibit a degrading enzyme, called plasmin, permitting this substance to be able to improve the resist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapeptide
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds. Examples of tetrapeptides are: * Tuftsin (L-threonyl-L-lysyl-L-prolyl-L-arginine) is a peptide related primarily to the immune system function. * Rigin (glycyl-L-glutaminyl-L-prolyl-L-arginine) is a tetrapeptide with functions similar to those of tuftsin. * Postin (Lys-Pro-Pro-Arg) is the N-terminal tetrapeptide of cystatin C and an antagonist of tuftsin. * Endomorphin-1 (H-Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (H-Tyr-Pro-Phe-Phe-NH2) a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrapeptide Structural Formulae V
A tetrapeptide is a peptide, classified as an oligopeptide, since it only consists of four amino acids joined by peptide bonds. Many tetrapeptides are pharmacologically active, often showing affinity and specificity for a variety of receptors in protein-protein signaling. Present in nature are both linear and cyclic tetrapeptides (CTPs), the latter of which mimics protein reverse turns which are often present on the surface of proteins and druggable targets. Tetrapeptides may be cyclized by a fourth peptide bond or other covalent bonds. Examples of tetrapeptides are: * Tuftsin (L-threonyl-L-lysyl-L-prolyl-L-arginine) is a peptide related primarily to the immune system function. * Rigin (glycyl-L-glutaminyl-L-prolyl-L-arginine) is a tetrapeptide with functions similar to those of tuftsin. * Postin (Lys-Pro-Pro-Arg) is the N-terminal tetrapeptide of cystatin C and an antagonist of tuftsin. * Endomorphin-1 (H-Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (H-Tyr-Pro-Phe-Phe-NH2) are pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclamide
Cyclamides are a class of oligopeptides produced by cyanobacteria algae strains such as '' Microcystis aeruginosa''. Some of them can be toxic. Cyclamides are cyclopeptides with either six or eight amino acids, some of which are modified from their natural proteinogenic form. They are typically characterized by thiazole and oxazole rings which are thought to be cysteine and threonine Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− ... derivatives, respectively. Cyclamides are biosynthesized through ribosomic pathways. See also * Cyanopeptolin * Microcystin References External links * {{Cyanotoxins Peptides Cyanotoxins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceruletide
Ceruletide (INN), also known as cerulein or caerulein, is a ten amino acid oligopeptide that stimulates smooth muscle and increases digestive secretions. Ceruletide is similar in action and composition to cholecystokinin. It stimulates gastric, biliary, and pancreatic secretion; and certain smooth muscle. It is used in paralytic ileus and as diagnostic aid in pancreatic malfunction. It is used to induce pancreatitis in experimental animal models. Ceruletide was discovered and its structure elucidated in 1967 by Australian and Italian scientists from dried skins of the Australian green tree frog (''Ranoidea caerulea'', formerly ''Hyla caerulea''). Its amino acid sequence is Pglu-Gln-Asp-Tyr O3HThr-Gly-Trp-Met-Asp-Phe-NH2. Induction of pancreatitis Ceruletide upregulates pancreatic acinar cell intercellular adhesion molecule-1 (ICAM-1) proteins through intracellular upregulation of NF-κB. Surface ICAM-1 in turn promotes neutrophil adhesion onto acinar cells enhancing pancr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanopeptolin
Cyanopeptolins (CPs) are a class of oligopeptides produced by Microcystis and Planktothrix algae strains, and can be neurotoxic. The production of cyanopeptolins occurs through nonribosomal peptides synthases (NRPS). Chemistry CPs are, in general, a six-residue peptide formed into a ring by a beta-lactone bridge, making them chemically depsipeptides (peptidolactones). The first position is usually threonine, which links to one or two residues via an ester bound on the beta-hydroxyl group; the third position is conserved to be 3-amino-6-hydroxy-2-piperidone (Ahp) or a derivative. All other positions are highly variable. There is not a single, unified nomenclature, for CPs. Names such as CP1020 and CP1138 refer to the molar mass. Others, such as aeruginopeptins, micropeptins, microcystilide, nostopeptins, and oscillapeptins, refer to the organism the substance is originally found in. Factors affecting production Increased water temperatures, because of climate change and eutrophic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L-glutamate and L-cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pancreatitis
Pancreatitis is a condition characterized by inflammation of the pancreas. The pancreas is a large organ behind the stomach that produces digestive enzymes and a number of hormone A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physio ...s. There are two main types, acute pancreatitis and chronic pancreatitis. Signs and symptoms of pancreatitis include epigastrium, pain in the upper abdomen, nausea, and vomiting. The pain often goes into the back and is usually severe. In acute pancreatitis, a fever may occur; symptoms typically resolve in a few days. In chronic pancreatitis, weight loss, steatorrhea, fatty stool, and diarrhea may occur. Complications may include infection, bleeding, diabetes mellitus, or problems with other organs. The two most common causes of acute pancreatitis ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amanita Phalloides
''Amanita phalloides'' ( ), commonly known as the death cap, is a deadly poisonous basidiomycete fungus and mushroom, one of many in the genus ''Amanita''. Originating in Europe but later introduced to other parts of the world since the late twentieth century, ''A. phalloides'' forms ectomycorrhizas with various broadleaved trees. In some cases, the death cap has been introduced to new regions with the cultivation of non-native species of oak, chestnut, and pine. The large fruiting bodies (mushrooms) appear in summer and autumn; the caps are generally greenish in colour with a white stipe and gills. The cap colour is variable, including white forms, and is thus not a reliable identifier. These toxic mushrooms resemble several edible species (most notably Caesar's mushroom and the straw mushroom) commonly consumed by humans, increasing the risk of accidental poisoning. Amatoxins, the class of toxins found in these mushrooms, are thermostable: they resist changes due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
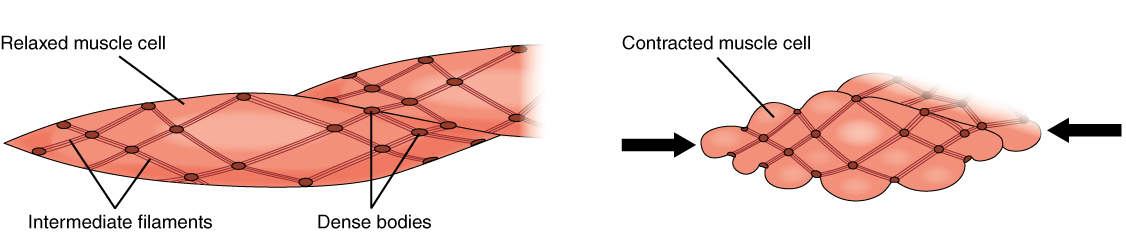
Smooth Muscle
Smooth muscle is one of the three major types of vertebrate muscle tissue, the others being skeletal and cardiac muscle. It can also be found in invertebrates and is controlled by the autonomic nervous system. It is non- striated, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It can be divided into two subgroups, ''single-unit'' and ''multi-unit'' smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium. Smooth muscle is found in the walls of hollow organs, including the stomach, intestines, bladder and uterus. In the walls of blood vessels, and lymph vessels, (excluding blood and lymph capillaries) it is known as vascular smooth muscle. There is smooth muscle in the tracts of the respiratory, urinary, and reproductive systems. In the eyes, the ciliary muscles, iris dilator muscle, and iris sphincter muscle are types of smooth muscles. The iris dilator and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholecystokinin
Cholecystokinin (CCK or CCK-PZ; from Greek ''chole'', "bile"; ''cysto'', "sac"; ''kinin'', "move"; hence, ''move the bile-sac (gallbladder)'') is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of pancreatic juice from the pancreas and bile from the gallbladder. History Evidence that the small intestine controls the release of bile was uncovered as early as 1856, when French physiologist Claude Bernard showed that when dilute acetic acid was applied to the orifice of the bile duct, the duct released bile into the duodenum. In 1903, the French physiologist showed that this reflex was not mediated by the nervous system. In 1904, the French physiologist Charles Fleig showed that the discharge of bile was mediated by a substance t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |