|
Nitrous Acid
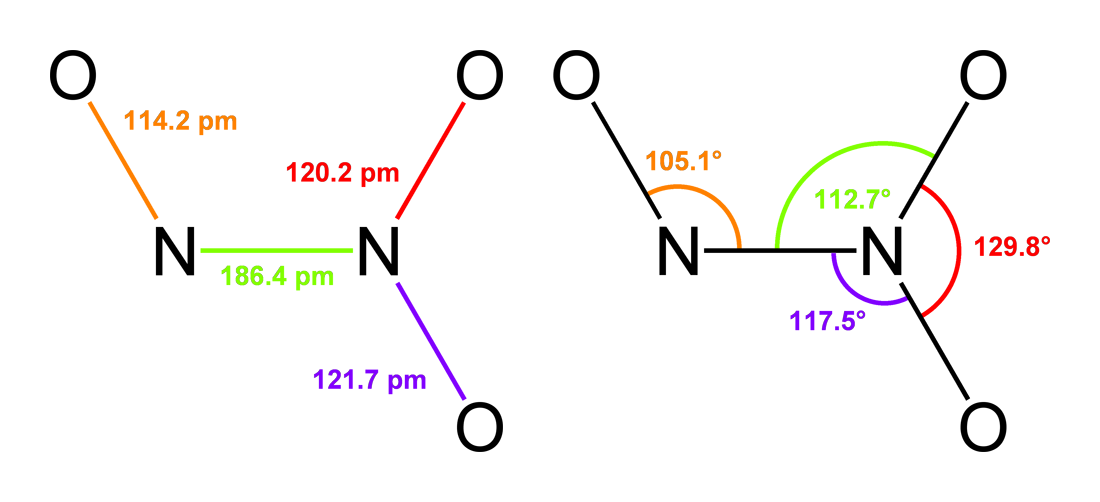
Nitrous acid (molecular formula ) is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite () salts. It was discovered by Carl Wilhelm Scheele, who called it " phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes. Structure In the gas phase, the planar nitrous acid molecule can adopt both a ''syn'' and an ''anti'' form. The ''anti'' form predominates at room temperature, and IR measurements indicate it is more stable by around 2.3 kJ/mol. p. 462. Image:Trans-nitrous-acid-2D-dimensions.png , Dimensions of the ''anti'' form(from the microwave spectrum) Image:Trans-nitrous-acid-3D-balls.png , Model of the ''anti'' form Image:Cis-nitrous-acid-3D-balls.png , ''syn'' form Preparation and decomposition Free, gaseous nitrous acid is unstable, rapidly disproportionating to nitric oxides: :2 HNO2 → NO2 + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
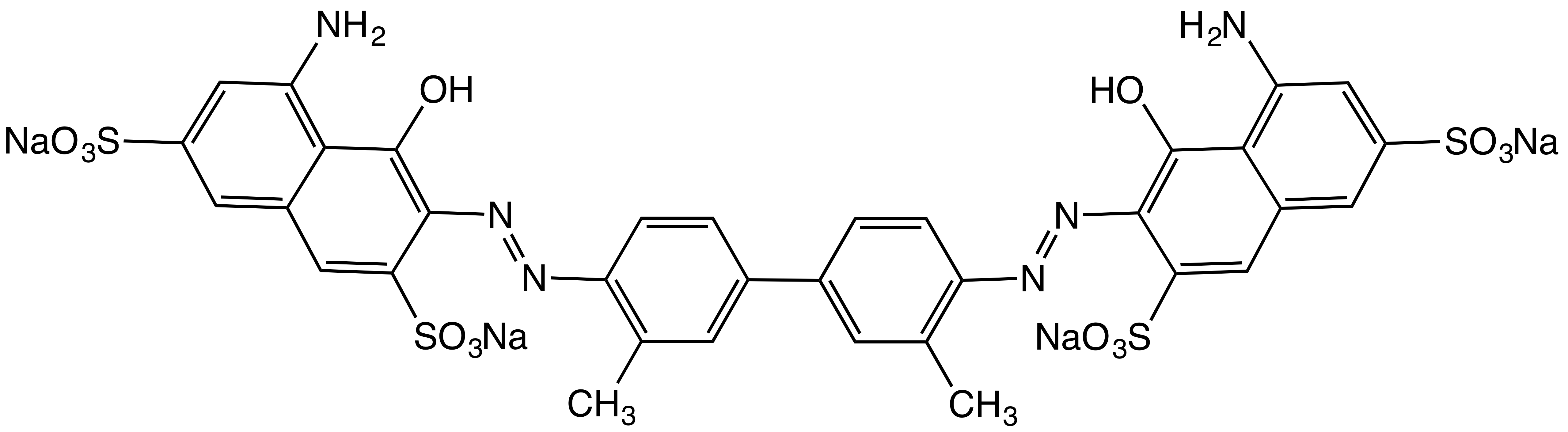
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liebermann Reagent
The Liebermann reagent named after Hungarian chemist Leo Liebermann (1852-1926) is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It is composed of a mixture of potassium nitrite and concentrated sulfuric acid. 1 g of potassium nitrite is used for every 10 mL of sulfuric acid. Potassium nitrite may also be substituted by sodium nitrite. It is used to test for cocaine, morphine, PMA and PMMA. The test is performed by scraping off a small amount of the substance and adding a drop of the reagent (which is initially clear and colorless). The results are analyzed by viewing the color of the resulting mixture, and by the time taken for the change in color to become apparent. See also *Drug checking * Liebermann–Burchard test * Dille–Koppanyi reagent *Folin's reagent *Froehde reagent *Mandelin reagent *Marquis reagent *Mecke reagent The Mecke reagent is used as a simple spot-test to presumptively identify alkaloids as well as other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dinitrogen Trioxide
Dinitrogen trioxide (also known as nitrous anhydride) is the inorganic compound with the formula . It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21°C (−6°F): : + Dinitrogen trioxide is only isolable at low temperatures (i.e., in the liquid and solid phases). In liquid and solid states, it has a deep blue color. At higher temperatures the equilibrium favors the constituent gases, with ''KD'' = 193 kPa (25°C). This compound is sometimes called "nitrogen trioxide", but this name properly refers to another compound, the (uncharged) nitrate radical . Structure and bonding Dinitrogen trioxide molecule contains an N–N bond. One of the numerous resonant structures of the molecule of dinitrogen trioxide is , which can be described as a nitroso group attached to a nitro group by a single bond between the two nitrogen atoms. This isomer is considered as the "anhydride" of the unstable nitrous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitrite
Sodium nitrite is an inorganic compound with the chemical formula . It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products. Uses Industrial chemistry The main use of sodium nitrite is for the industrial production of organonitrogen compounds. It is a reagent for conversion of amines into diazo compounds, which are key precursors to many dyes, such as diazo dyes. Nitroso compounds are produced from nitrites. These are used in the rubber industry. It is used in a variety of metallurgical applications, for phosphatizing and detinning. Sodium nitrite is an effective corrosion inhibitor and is used as an additive in industrial greases, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral Acid
A mineral acid (or inorganic acid) is an acid derived from one or more inorganic compounds, as opposed to organic acids which are acidic, organic compounds. All mineral acids form hydrogen ions and the conjugate base when dissolved in water. Characteristics Commonly used mineral acids are sulfuric acid (H2SO4), hydrochloric acid (HCl) and nitric acid (HNO3); these are also known as bench acids. Mineral acids range from superacids (such as perchloric acid) to very weak ones (such as boric acid). Mineral acids tend to be very soluble in water and insoluble in organic solvents. Mineral acids are used in many sectors of the chemical industry as feedstocks for the synthesis of other chemicals, both organic and inorganic. Large quantities of these acids—especially sulfuric acid, nitric acid, and hydrochloric acid—are manufactured for commercial use in large plants. Mineral acids are also used directly for their corrosive properties. For example, a dilute solution of hydrochl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cambridge University Press
Cambridge University Press was the university press of the University of Cambridge. Granted a letters patent by King Henry VIII in 1534, it was the oldest university press in the world. Cambridge University Press merged with Cambridge Assessment to form Cambridge University Press and Assessment under Queen Elizabeth II's approval in August 2021. With a global sales presence, publishing hubs, and offices in more than 40 countries, it published over 50,000 titles by authors from over 100 countries. Its publications include more than 420 academic journals, monographs, reference works, school and university textbooks, and English language teaching and learning publications. It also published Bibles, runs a bookshop in Cambridge, sells through Amazon, and has a conference venues business in Cambridge at the Pitt Building and the Sir Geoffrey Cass Sports and Social Centre. It also served as the King's Printer. Cambridge University Press, as part of the University of Cambridge, was a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the " Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxides
In atmospheric chemistry, is shorthand for nitric oxide () and nitrogen dioxide (), the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone. gases are usually produced from the reaction between nitrogen and oxygen during combustion of fuels, such as hydrocarbons, in air; especially at high temperatures, such as in car engines. In areas of high motor vehicle traffic, such as in large cities, the nitrogen oxides emitted can be a significant source of air pollution. gases are also produced naturally by lightning. does not include nitrous oxide (), a fairly inert oxide of nitrogen that contributes less severely to air pollution, notwithstanding its involvement in ozone depletion and high global warming potential. is the class of compounds comprising and the compounds produced from the oxidation of which include nitric acid, nitrous acid (HONO), dinitroge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ball-and-stick Model
In chemistry, the ball-and-stick model is a molecular model of a chemical substance which displays both the Molecular geometry, three-dimensional position of the atoms and the chemical bond, bonds between them. The atoms are typically represented by sphere (geometry), spheres, connected by rods which represent the bonds. Double bond, Double and triple bonds are usually represented by two or three curved rods, respectively, or alternately by correctly positioned sticks for the sigma bond, sigma and pi bonds. In a good model, the angles between the rods should be the same as the Bond angle, angles between the bonds, and the distances between the centers of the spheres should be proportional to the distances between the corresponding atomic nucleus, atomic nuclei. The chemical element of each atom is often indicated by the sphere's color. In a ball-and-stick model, the radius of the spheres is usually much smaller than the rod lengths, in order to provide a clearer view of the atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |