|
Nitroreductase
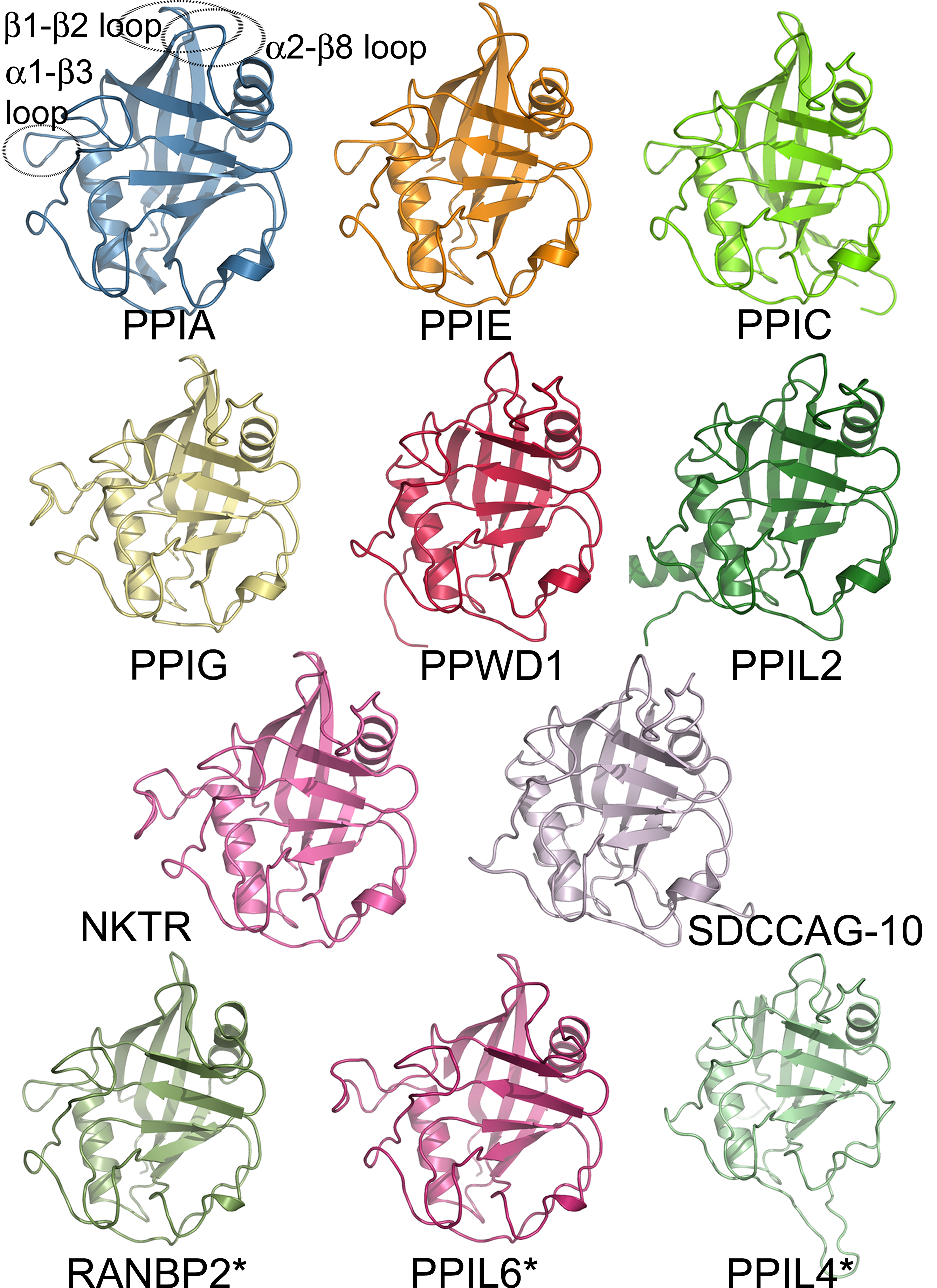
Nitroreductases are a family of evolutionarily related proteins involved in the reduction of nitrogen-containing compounds, including those containing the nitro functional group. Members of this family utilise flavin mononucleotide as a cofactor and are often found to be homodimers. Members of this family include oxygen-insensitive NAD(P)H nitroreductase (flavin mononucleotide-dependent nitroreductase) ( 6,7-dihydropteridine reductase) () and NADH dehydrogenase (). A number of these proteins are described as oxidoreductases. They are primarily found in bacterial lineages though a number of eukaryotic homologs have been identified: '' C. elegans'' , '' D. melanogaster'' , , mouse and human . This protein is not found in photosynthetic eukaryotes. The sequences containing this entry in photosynthetic organisms are possible false positives. The nitroreductase of ''Enterobacter cloacae'' was identified by Bryant and Deluca in a strain isolated from a munitions facility, on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Family
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. Sequence similarity (usually amino-acid sequence) is one of the most common indicators of homology, or common evolutionary ancestry. Some frameworks for evaluating the significance of similarity between sequences use sequence alignment methods. Proteins that do not share a common ancestor are unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein families. Families are sometimes grouped together into larger clades called superfamilies based on st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitro Compound
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid. Synthesis Preparation of aromatic nitro compounds Aromatic nitro compounds are typically synthesized by nitration. Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (), which is the electrophile: + The nitration product produced on the largest scale, by f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavin Mononucleotide
Flavin mononucleotide (FMN), or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as the prosthetic group of various oxidoreductases, including NADH dehydrogenase, as well as a cofactor in biological blue-light photo receptors. During the catalytic cycle, various oxidoreductases induce reversible interconversions between the oxidized (FMN), semiquinone (FMNH•), and reduced (FMNH2) forms of the isoalloxazine core. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. In its role as blue-light photo receptor, (oxidized) FMN stands out from the 'conventional' photo receptors as the signaling state and not an E/Z isomerization. It is the principal form in which riboflavin is found in cells and tissues. It requires more energy to produce, but is more soluble than riboflavin. In cells, FMN occurs freely circulating but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst (a catalyst is a substance that increases the rate of a chemical reaction). Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound. Cofactors can be classified into two types: inorganic ions and complex organic molecules called coenzymes. Coenzymes are mostly derived from vitamins and other organic essential nutrients in small amounts. (Some scientists limit the use of the term "cofactor" for inorganic substances; both types are included here.) Coenzymes are further divided into two types. The first is called a " prosthetic group", which consists of a coenzyme that is tightly (or even covalently and, therefore, permanently) bound to a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
6,7-dihydropteridine Reductase
In enzymology, 6,7-dihydropteridine reductase (, also Dihydrobiopterin reductase) is an enzyme that catalyzes the chemical reaction : 5,6,7,8-tetrahydropteridine + NAD(P)+ \rightleftharpoons 6,7-dihydropteridine + NAD(P)H + H+ The four substrates for this enzyme are a 6,7-dihydropteridine ( dihydrobiopterin), NADH, NADPH, and H+ and its three products are 5,6,7,8-tetrahydropteridine ( tetrahydrobiopterin), NAD+, and NADP+ This enzyme participates in folate biosynthesis. In the human genome, the enzyme is encoded by the QDPR gene. Nomenclature This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-NH group of donors with NAD+ or NADP+ as acceptor. The systematic name of this enzyme class is 5,6,7,8-tetrahydropteridine:NAD(P)+ oxidoreductase. Other names in common use include 6,7-dihydropteridine:NAD(P)H oxidoreductase, DHPR, NAD(P)H:6,7-dihydropteridine oxidoreductase, NADH-dihydropteridine reductase, NADPH-dihydropteridine reductas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH Dehydrogenase
NADH dehydrogenase is an enzyme that converts nicotinamide adenine dinucleotide (NAD) from its reduced form (NADH) to its oxidized form (NAD+). Members of the NADH dehydrogenase family and analogues are commonly systematically named using the format ''NADH:acceptor oxidoreductase''. The chemical reaction these enzymes catalyze is generally represented with the following equation: : NADH + H+ + acceptor NAD+ + reduced acceptor NADH dehydrogenase is a flavoprotein that contains iron-sulfur centers. NADH dehydrogenase is used in the electron transport chain for generation of ATP. The EC term NADH dehydrogenase (quinone) (EC 1.6.5.11) is defined for NADH dehydrogenases that use a quinone The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with ... (excluding ubiquinone) as the acceptor. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caenorhabditis Elegans
''Caenorhabditis elegans'' () is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a Hybrid word, blend of the Greek ''caeno-'' (recent), ''rhabditis'' (rod-like) and Latin ''elegans'' (elegant). In 1900, Émile Maupas, Maupas initially named it ''Rhabditidae, Rhabditides elegans.'' Günther Osche, Osche placed it in the subgenus ''Caenorhabditis'' in 1952, and in 1955, Ellsworth Dougherty, Dougherty raised ''Caenorhabditis'' to the status of genus. ''C. elegans'' is an unsegmented pseudocoelomate and lacks respiratory or circulatory systems. Most of these nematodes are hermaphrodites and a few are males. Males have specialised tails for mating that include spicule (nematode), spicules. In 1963, Sydney Brenner proposed research into ''C. elegans,'' primarily in the area of neuronal development. In 1974, he began research into the molecular biology, molecular and developmental ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster
''Drosophila melanogaster'' is a species of fly (an insect of the Order (biology), order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly", or "banana fly". In the wild, ''D. melanogaster'' are attracted to rotting fruit and fermenting beverages, and are often found in orchards, kitchens and pubs. Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, ''D. melanogaster'' continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and Life history theory, life history evolution. ''D. melanogaster'' was the first animal to be Fruit flies in space, launched into space in 1947. As of 2017, six Nobel Prizes have been awarded to drosophilists for their work using the insect. ''Drosophila melanogaster'' is typically used in research owing to its rapid life cycle, relatively simple genetics with on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioremediation
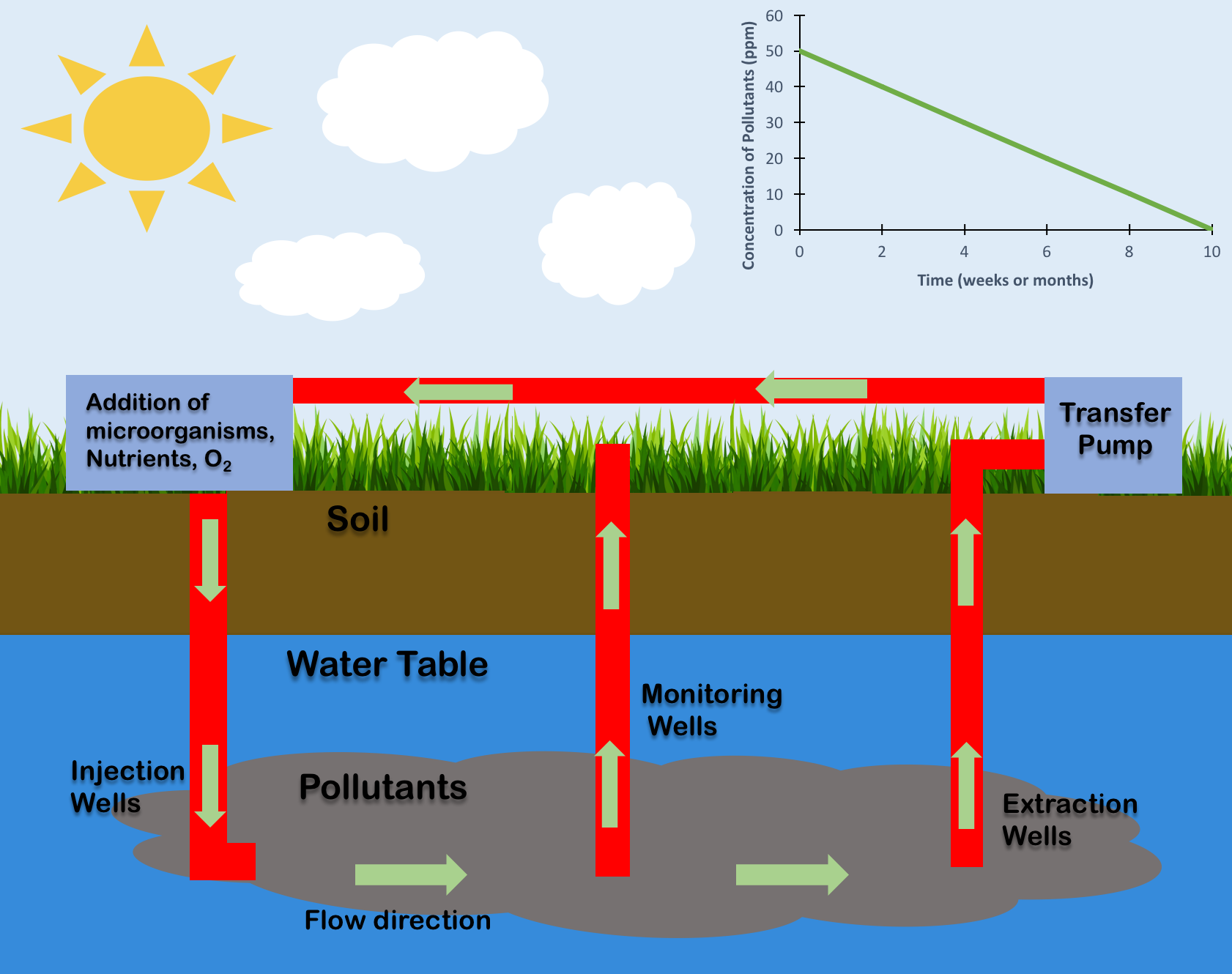
Bioremediation broadly refers to any process wherein a biological system (typically bacteria, microalgae, fungi in mycoremediation, and plants in phytoremediation), living or dead, is employed for removing environmental pollutants from air, water, soil, fuel gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer advantages as it aims to be sustainable, eco-friendly, cheap, and scalable. This technology is rarely implemented however because it is slow or inefficient. Most bioremediation is inadvertent, involving native organisms. Research on bioremediation is heavily focused on stimulating the process by inoculation of a polluted site with organisms or supplying nutrients to promote their growt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cob(II)yrinic Acid A,c-diamide Reductase
In enzymology, a cob(II)yrinic acid a,c-diamide reductase () is an enzyme that catalysis, catalyzes the chemical reaction 2 cob(I)yrinic acid a,c-diamide + FMN + 3 H+ \rightleftharpoons 2 cob(II)yrinic acid a,c-diamide + FMNH2 The three substrate (biochemistry), substrates of this enzyme are cob(I)yrinic acid a,c-diamide, flavin mononucleotide, and hydrogen ion, H+; its two product (chemistry), products are cob(II)yrinic acid a,c-diamide and FMNH2. Classification This enzyme belongs to the family of oxidoreductases, specifically those oxidizing metal ion with a flavin as acceptor. Nomenclature The List of enzymes, systematic name of this enzyme class is cob(I)yrinic acid-a,c-diamide:FMN oxidoreductase. This enzyme is also called CobR and cob(II)yrinic acid-a,c-diamide:FMN oxidoreductase (incorrect). Biological role This enzyme is part of the biosynthetic pathway to cobalamin (vitamin B12) in bacteria. See also * Cobalamin biosynthesis References * * EC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodotyrosine Deiodinase
Iodotyrosine deiodinase, also known as iodotyrosine dehalogenase 1, is a type of deiodinase enzyme that scavenges iodide by removing it from iodinated tyrosine residues in the thyroid gland. These iodinated tyrosines are produced during thyroid hormone biosynthesis. The iodide that is scavenged by iodotyrosine deiodinase is necessary to again synthesize the thyroid hormones. After synthesis, the thyroid hormones circulate through the body to regulate metabolic rate, protein expression, and body temperature. Iodotyrosine deiodinase is thus necessary to keep levels of both iodide and thyroid hormones in balance. Dehalogenation in aerobic organisms is usually done through Redox, oxidation and hydrolysis; however, iodotyrosine deiodinase uses reductive dehalogenation. Iodotyrosine deiodinase and iodothyronine deiodinase have been determined as the only two known enzymes to catalyze reductive dehalogenation in mammals. Although these two enzymes perform similar functions, they are st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |